Medicine:Antisynthetase syndrome
| Anti-synthetase syndrome | |
|---|---|
| Other names | Anti-Jo1 syndrome, AS syndrome, ASS. |
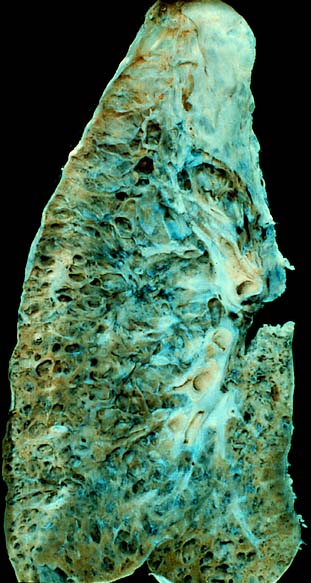 | |
| End-stage interstitial lung disease. | |
| Specialty | Immunology |
| Symptoms | Fever, myositis, polyarthritis, interstitial lung disease, mechanic's hands, and Raynaud phenomenon.[1] |
| Differential diagnosis | Dermatomyositis, Immune-mediated necrotizing myopathy, and Polymyositis.[2] |
| Treatment | Glucocorticosteroids, immunosuppressive medications.[1] |
| Frequency | 1/25,000 - 33,000 worldwide.[2] |
Antisynthetase syndrome (ASS) is a multisystematic autoimmune disease associated with inflammatory myositis, interstitial lung disease, and antibodies directed against various synthetases of aminoacyl-transfer RNA.[3] Other common symptoms include mechanic's hands, Raynaud's phenomenon, arthritis, and fever.[4]
It is still unknown what causes interstitial lung disease associated with antisynthetase syndrome.[5] Many antisynthetase antibodies have been reported with anti-Jo1 being the most prevalent.[6] Pulmonary involvement is an important factor of morbidity and mortality with Antisynthetase syndrome, affecting 70-100% of patients.[7]
Antisynthetase syndrome is diagnosed by a combination of radiologic features, clinical criteria, and identification of aminoacyl tRNA synthetase antibodies.[8] Immunosuppressive medications such as mycophenolate mofetil, azathioprine, and tacrolimus are often used alongside corticosteroids to manage myositis and other pulmonary symptoms.[9]
It is believed that the mortality rate for antisynthetase syndrome is significantly higher than that of the general population.[10] The estimated cumulative ten-year survival rate for patients with different antisyntetase antibodies is 76.8%.[11]
Antisynthetase syndrome is estimated by Orphanet to affect 1-3 people per 100,000 worldwide; however, precise data on the disease's prevalence is not available.[12] Antisynthetase syndrome is more common in women.[13]
Signs and symptoms
67 to 100% of those with antisynthetase syndrome have interstitial lung disease[13] with dyspnea and cough being the most common symptoms.[14] Pulmonary symptoms may present early on, alongside other symptoms or they may manifest later in the progression of antisynthetase syndrome.[15] A recent study showed that pleural involvement is often associated with antisynthetase syndrome, reporting that 42.2% patients with antisynthetase syndrome had pleural effusions.[16]
91% of patients with antisynthetase syndrome displayed myopathic muscular weakness.[13] Myositis is more commonly seen in those with anti-Jo1 than other antibodies.[17] Myopathy in antisynthetase syndrome can range from subclinical to substantial proximal weakness causing difficulties climbing stairs, reaching overhead cupboards, or getting up from a seated position.[18] 30.4 to 88.9% of those with antisynthetase syndrome report persistent muscular tenderness and myalgia.[19][20]
In antisynthetase syndrome, arthritis is commonly described as a symmetrical, non-erosive polyarthritis of the small hands and feet that can sometimes mimic CTD-associated, rheumatoid, and seronegative inflammatory arthritis.[21] Arthritis is non-specific and occurs in about 18 to 55% of Idiopathic inflammatory myopathies.[21] Arthritis is the presenting symptom in about 24-66% of patients which can often lead to diagnostic delays.[3] Hand X-ray lesions such as erosions, periarticular calcifications, and subluxations are seen in about 50% of patients.[22]
Raynaud's phenomenon is an episodic vasospasm of the toes and fingers usually precipitated by exposure to cold temperatures.[23] Raynaud's phenomenon can be primary or secondary to another disease.[24]
The term "mechanics hands" describes the roughening and cracking of the skin on the lateral sides of the fingers, usually the thumb's ulnar border and the index finger's radial border. Interface psoriasiform dermatitis is seen on biopsy.[25]
Dermatomyositis-like rashes are seen in 32-44% of those with antisynthetase syndrome. This includes heliotropic rash, Gottron papules, and lesions of the psoriasiform type that span the hand's dorsum and resemble the morphology of mechanics hands.[26]
Fever is reported in 21 to 66% of those with antisynthetase syndrome.[27][28]
Cardiac involvement such as pulmonary hypertension[29] and myocarditis[30] have also been reported. Other reported manifestations include sicca symptoms and dysphagia.[31]
Pathogenesis
It is postulated that autoantibodies are formed against aminoacyl-tRNA synthetases. The synthetases may be involved in recruiting antigen-presenting and inflammatory cells to the site of muscle or lung injury. The specific molecular pathway of the process awaits elucidation.[18]
Antisynthetase antibodies
The most common antibody is "Anti-Jo-1" named after John P, a patient with polymyositis and interstitial lung disease detected in 1980.[32] This anti-histidyl tRNA Synthetase antibody is commonly seen in patients with pulmonary manifestations of the syndrome. The following are other possible antibodies that may be seen in association with antisynthetase syndrome: Anti-PL-7, Anti-PL-12, Anti-EJ, Anti-OJ, Anti-KS, Anti-Zo, Anti-Ha (YRS, Tyr).[33]
Diagnosis
The diagnostic criteria for antisynthetase syndrome include one or more anti-synthetase antibodies as well inflammatory polyarthritis that affects the small joints, inflammatory myopathy, or interstitial lung disease.[34]
A wide range of associated autoantibodies and myositis-specific antibodies have been found in those with idiopathic inflammatory myopathies and are often tested when evaluating idiopathic inflammatory myopathy.[35] Anti-synthetase antibodies specific to myositis are the defining feature of anti-synthetase syndrome. Anti-histidyl-tRNA synthetase and anti-Jo-1 are the most commonly found anti-synthetase antibodies. Less common antibodies include anti-EJ, anti-OJ, anti-PL12, and anti-PL7.[9]
The diagnosis of idiopathic interstitial lung disease can also involve testing for other myositis-specific autoantibodies such as anti-MDA5 autoantibodies,[36] anti-Mi-2 autoantibodies, and anti-signal recognition particle autoantibodies.[37] Autoantibodies often tested include anti-Ro/SSA,[38] anti-nuclear antibody, anti-U1-RNP, anti-Ku, and anti-PM-Scl.[37] Although anti-La/SSB and anti-Ro/SSA are often associated with Sjogren's syndrome they have been associated with antisynthetase syndrome and severe fibrotic idiopathic interstitial lung disease by high resolution computed tomography.[39]
High-resolution computed tomography is common in the management and workup of interstitial lung disease. One study showed that reticulation, ground glass opacities, and traction bronchiectasis are the most common CT scan features of antisynthetase syndrome.[40] The presence of organizing pneumonia and non-specific interstitial pneumonia is somewhat common in antisynthetase syndrome.[41]
Lung biopsy is rarely performed when evaluating patients with antisynthetase syndrome since the diagnosis is usually made by a combination of symptoms, physical examination, high-resolution computed tomography findings, pulmonary function tests, and serologic data.[9] One study that analyzed the histopathologic findings using lung biopsies in patients with anti-PL7 antibodies and anti-Jo-1 antibodies found that 35% of patients had usual interstitial pneumonia and 50% had diffuse alveolar damage.[42]
Because interstitial lung disease is the main finding and myositis is often mild, muscle biopsy is rarely performed.[9] Although muscle biopsy is often helpful in other forms of idiopathic inflammatory myopathies, there is currently no evidence to support the use of muscle biopsy when diagnosing antisynthetase syndrome.[43]
Treatment
The first line of treatment for idiopathic inflammatory myopathies is usually corticosteroids. However, when corticosteroids are tapered down there is often lung disease recurrence. Other immunosuppressive drugs can be added as corticosteroid-sparing agents or for refractory lung and muscle disease. Cyclophosphamide, rituximab, tacrolimus, mycophenolate mofetil, and azathioprine are often used alongside corticosteroids however there is no agreement as to which medication is preferred.[9]
No controlled trials have been preformed to prove the superiority of corticosteroids compared to other immunosuppressive drugs in the treatment of antisynthetase syndrome. Those with antisynthetase syndrome often need multimodality treatment with frequent lung function monitoring.[44]
By inhibiting purine synthesis along with incorporating metabolites into DNA, azathioprine reduces immune function by preventing replication, especially of immune cells. Azathioprine is often used in the treatment of inflammatory myopathy and interstitial lung disease, especially when there is no response to corticosteroids. Azathioprine is typically used in combination with prednisone as a maintenance therapy.[45]
Mycophenolate mofetil changes purine synthesis to prevent lymphocytes from multiplying. It is often used after transplants to avoid rejection and is also used to treat connective tissue disorders. Studies have shown it's efficacy and safety in patients with connective tissue disorders and interstitial lung disease.[46]
Tacrolimus and cyclosporine, two calcineurin inhibitors, work against the immune system by inhibiting T cells. One study showed that when patients with anti-Jo-1 antibodies received cyclosporine they displayed improved diffusing capacity for carbon monoxide and forced vital capacity.[47] A 1999 study reported that tacrolimus successfully treats polymyositis with interstitial lung disease.[48] Since then, many studies have suggested that tacrolimus is successful in treating patients with connective tissue disorders and inflammatory myopathy related interstitial lung disease.[49] It has also been used in combination with other immunosuppressive drugs and corticosteroids.[50] In patients with antisynthetase syndrome related interstitial lung disease tacrolimus has been demonstrated to improve muscle symptoms and lung function.[51]
Rituximab is a monoclonal antibody that specifically targets B-lymphocytes' CD20 surface antigen. One case series reported increased forced vital capacity in patients with antisynthetase syndrome-related interstitial lung disease who were treated with rituximab.[52] Another study showed improvements in lung function and/or respiratory symptoms in patients who has interstitial lung disease due to connective tissue disorders. Half of the patients had anti-Jo-1 antibodies.[53]
Cyclophosphamide is a cytotoxic agent that inhibits proliferation by alkylating DNA. It is usually only used to treat severe inflammatory myopathy and interstitial lung disease, especially acute respiratory distress syndrome, due to the severe nature of many of the possible side effects. Numerous case series have shown its effectiveness in treating inflammatory myopathy with and without interstitial lung disease.[54][55]
Although its precise mode of action is unknown, intravenous immunoglobulin (IVIG), a blood product made up of donated blood's pooled immunoglobulin G antibodies, is used to treat a variety of autoimmune and immune deficiency diseases. Patients with dermatomyositis who received IVIG showed a significant improvement in their symptoms and muscle strength in a randomized, placebo-controlled trial of the treatment.[56] Another case report suggests that IVIG can be effective in treating interstitial lung disease related to inflammatory myopathy.[57]
A case study has reported that the use of CD19-targeting CAR T cell therapy (originally used to treat lymphoma and leukemia) in a patient with refractory anti-synthetase syndrome had complete clinical remission during 200 days of follow-up post-infusion when the patient received with chimeric antigen receptor (CAR) T cells that recognize CD19+ B cells.[58] This shows that CD19-targeting CAR T cells could be a new therapy for patients who do not respond to the first-line immunosuppressive drugs.[59]
If there is esophageal involvement, gastric intubation or percutaneous endoscopic gastrostomy may be required.[60] Owing to severe interstitial lung disease, 14% of those with anti-Jo-1 or anti-PL-7 antibodies need oxygen therapy.[17] Rarely, medication is not successful and a lung transplant is necessary.[61] Patients with myositis should be advised to engage in individually tailored physical exercise because it lowers activity restrictions and has an anti-inflammatory effect.[62]
Outlook
Antisynthetase syndrome mortality is thought to be substantially higher than that of the general population.[10] Patients who had various antisyntetase antibodies had an estimated cumulative ten-year survival rate of 76.8%.[11] Respiratory complications, infectious diseases like pneumonia, cancer,[60] cardiovascular disorders,[63] severe myositis, and interstitial lung disease are the main causes of death amongst anti Jo-1-positive patients.[10]
Risk factors for severe antisynthetase syndrome include calcinosis, esophageal involvement, cancer, interstitial lung disease without myositis, severe respiratory involvement, and older age at the time of diagnosis.[10] Muscle weakness and arthritis at the point of diagnosis have been noted as favorable outcome measures.[63]
One study reported that anti-PL-12 and anti-PL-7 antibodies were associated with a worse prognosis.[29] A large portion of deaths occurred during the first year of onset and mostly involved those with anti-PL-7.[10][63] Interstitial lung disease often led to deadly prognosis in comparison to anti-Jo1-positive patients with interstitial lung disease. Those with anti-Jo1 antibodies had less remission of myositis and more relapses than those with anti-PL-12 and anti-PL-7 antibodies.[64] Anti-Jo-1 and anti-Ro52 antibodies co-occurring were linked to an increased risk of neoplasm, a symptomatic severe variant of interstitial lung disease, myositis, and arthritis exacerbation.[20]
Although dermatomyositis has long been linked to an elevated risk of cancer, there is insufficient data to support this association when evaluating individuals who have antisynthetase syndrome for potential cancer.[9] The first documented case of antisynthetase syndrome linked to cancer occurred in 2008, involving a 59-year-old patient diagnosed with colon adenocarcinoma.[65] There have been numerous case reports along with case series since, with significantly different prevalence rates up to about 14%.[66][67] The frequency of cancer within three years of the anti-synthetase syndrome diagnosis was only 1.7% in a more comprehensive retrospective analysis involving 233 patients with the condition, which the authors claim was not significantly different from the population as a whole.[17] Age-appropriate cancer screening should be followed by healthcare providers for these patients, according to these studies, but there is no evidence to support further or non-guideline-based assessments. It's interesting to note that case reports have shown that treating the underlying cancer can improve myositis as well as interstitial lung disease, which supports the theory that idiopathic inflammatory myopathy may be a paraneoplastic syndrome.[68]
Epidemiology
Orphanet estimates that the global rate of antisynthetase syndrome is 1–9/100 000; however, exact information regarding the disease's prevalence is not available.[12] 11.1%[69] to 39.19% of those with idiopathic inflammatory myopathy have antisynthetase antibodies.[70] The EuroMyositis registry indicates that antisynthetase syndrome is more common than immune-mediated necrotizing myopathy and sporadic inclusion body myositis, but less common than dermatomyositis and polymyositis. Similar to other subtypes of idiopathic inflammatory myopathy, except for sporadic inclusion body myositis, antisynthetase syndrome is more common in women, with an estimated female-to-male ratio of roughly 7:3. In patients with dermatomyositis and polymyositis, the mean age at disease onset is 48 years, which is older than in patients with immune-mediated necrotizing myopathy and sporadic inclusion body myositis.[13]
References
- ↑ 1.0 1.1 "Antisynthetase syndrome". https://dermnetnz.org/topics/antisynthetase-syndrome.
- ↑ 2.0 2.1 "Antisynthetase syndrome associated myositis". May 26, 2021. https://www.pathologyoutlines.com/topic/muscleantisynthetase.html.
- ↑ 3.0 3.1 "Serum Jo-1 Autoantibody and Isolated Arthritis in the Antisynthetase Syndrome: Review of the Literature and Report of the Experience of AENEAS Collaborative Group". Clinical Reviews in Allergy & Immunology (Springer Science and Business Media LLC) 52 (1): 71–80. February 2017. doi:10.1007/s12016-016-8528-9. PMID 26782036. https://link.springer.com/article/10.1007/s12016-016-8528-9. Retrieved December 4, 2023.
- ↑ "Clinical profile and treatment outcomes in antisynthetase syndrome: a tertiary centre experience". Rheumatology Advances in Practice (Oxford University Press (OUP)) 5 (Suppl 2): ii10–ii18. November 2021. doi:10.1093/rap/rkab054. PMID 34755025.
- ↑ Zhao, Na; Jiang, Wei; Wu, Hongliang; Wang, Ping; Wang, Xiaoni; Bai, Yu; Li, Yao; Tang, Yanchun et al. (August 10, 2022). "Clinical features, prognostic factors, and survival of patients with antisynthetase syndrome and interstitial lung disease". Frontiers in Immunology (Frontiers Media SA) 13. doi:10.3389/fimmu.2022.872615. ISSN 1664-3224. PMID 36032132.
- ↑ "Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes". The Quarterly Journal of Medicine (Oxford University Press (OUP)) 77 (282): 1019–1038. October 1990. doi:10.1093/qjmed/77.1.1019. PMID 2267280. https://academic.oup.com/qjmed/article-abstract/77/1/1019/1531796?redirectedFrom=fulltext&login=false. Retrieved December 4, 2023.
- ↑ "Antisynthetase syndrome with anti-Jo1 antibodies in 48 patients: pulmonary involvement predicts disease-modifying antirheumatic drug use". The Journal of Rheumatology 39 (9): 1835–1839. September 2012. doi:10.3899/jrheum.111604. PMID 22859355.
- ↑ "A multidisciplinary approach to the diagnosis of antisynthetase syndrome". Frontiers in Medicine (Frontiers Media SA) 9: 959653. September 14, 2022. doi:10.3389/fmed.2022.959653. PMID 36186825.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 "The Diagnosis and Treatment of Antisynthetase Syndrome". Clinical Pulmonary Medicine (Ovid Technologies (Wolters Kluwer Health)) 23 (5): 218–226. September 2016. doi:10.1097/cpm.0000000000000171. PMID 27594777.
- ↑ 10.0 10.1 10.2 10.3 10.4 Trallero-Araguás, Ernesto; Grau-Junyent, Josep María; Labirua-Iturburu, Anne; García-Hernández, Francisco José; Monteagudo-Jiménez, Manuel; Fraile-Rodriguez, Guadalupe; Les-Bujanda, Iñigo; Rodriguez-Carballeira, Mónica et al. (2016). "Clinical manifestations and long-term outcome of anti-Jo1 antisynthetase patients in a large cohort of Spanish patients from the GEAS-IIM group". Seminars in Arthritis and Rheumatism (Elsevier BV) 46 (2): 225–231. doi:10.1016/j.semarthrit.2016.03.011. ISSN 0049-0172. PMID 27139168. https://pubmed.ncbi.nlm.nih.gov/27139168/. Retrieved December 4, 2023.
- ↑ 11.0 11.1 Shi, Jingli; Li, Shanshan; Yang, Hanbo; Zhang, Yamei; Peng, Qinglin; Lu, Xin; Wang, Guochun (May 1, 2017). "Clinical Profiles and Prognosis of Patients with Distinct Antisynthetase Autoantibodies". The Journal of Rheumatology 44 (7): 1051–1057. doi:10.3899/jrheum.161480. ISSN 0315-162X. PMID 28461650. https://pubmed.ncbi.nlm.nih.gov/28461650/. Retrieved December 4, 2023.
- ↑ 12.0 12.1 "Orphanet: Antisynthetase syndrome". December 4, 2023. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=81.
- ↑ 13.0 13.1 13.2 13.3 "The EuroMyositis registry: an international collaborative tool to facilitate myositis research". Annals of the Rheumatic Diseases 77 (1): 30–39. January 2018. doi:10.1136/annrheumdis-2017-211868. PMID 28855174.
- ↑ "New Insights into Antisynthetase Syndrome". Maedica (Amaltea Medical, Editura Magister) 11 (2): 130–135. June 2016. PMID 28461832.
- ↑ "The clinical phenotype associated with antisynthetase autoantibodies". Reumatologia (Termedia Sp. z.o.o.) 58 (1): 4–8. February 28, 2020. doi:10.5114/reum.2020.93505. PMID 32322117.
- ↑ "Antisynthetase Syndrome: Prevalence of Serositis in Autoantibody Subsets". Arthritis Rheumatol. 70 (9). October 21, 2018. https://acrabstracts.org/abstract/antisynthetase-syndrome-prevalence-of-serositis-in-autoantibody-subsets/. Retrieved 1 December 2023.
- ↑ 17.0 17.1 17.2 "Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity". Autoimmunity Reviews (Elsevier BV) 12 (2): 210–217. December 2012. doi:10.1016/j.autrev.2012.06.006. PMID 22771754. https://www.sciencedirect.com/science/article/abs/pii/S1568997212001243.
- ↑ 18.0 18.1 "Antisynthetase syndrome: not just an inflammatory myopathy". Cleveland Clinic Journal of Medicine 80 (10): 655–666. October 2013. doi:10.3949/ccjm.80a.12171. PMID 24085811.
- ↑ "Antisynthetase syndrome — much more than just a myopathy". Seminars in Arthritis and Rheumatism (Elsevier BV) 51 (1): 72–83. February 2021. doi:10.1016/j.semarthrit.2020.09.020. PMID 33360231. https://pubmed.ncbi.nlm.nih.gov/33360231/. Retrieved December 4, 2023.
- ↑ 20.0 20.1 "Short-term and long-term outcome of anti-Jo1-positive patients with anti-Ro52 antibody". Seminars in Arthritis and Rheumatism (Elsevier BV) 41 (6): 890–899. June 2012. doi:10.1016/j.semarthrit.2011.09.008. PMID 22078416. https://pubmed.ncbi.nlm.nih.gov/22078416/. Retrieved December 4, 2023.
- ↑ 21.0 21.1 "Arthritis in Idiopathic Inflammatory Myopathies". Current Rheumatology Reports (Springer Science and Business Media LLC) 21 (12): 70. December 2019. doi:10.1007/s11926-019-0878-x. PMID 31813070. https://pubmed.ncbi.nlm.nih.gov/31813070/. Retrieved December 4, 2023.
- ↑ "AB0742 Hand x-ray lesions are frequent in antisynthetase syndrome patients with arthralgia and increase with the radiographic follow-up, whatever the extra-articular features and the serotype of antisynthetase syndrome". BMJ Publishing Group Ltd and European League Against Rheumatism. 2018. doi:10.1136/annrheumdis-2018-eular.4602. https://ard.bmj.com/content/77/Suppl_2/1509.1. Retrieved December 1, 2023.
- ↑ "Raynaud's phenomenon". Lancet (Elsevier BV) 357 (9273): 2042–2048. June 2001. doi:10.1016/s0140-6736(00)05118-7. PMID 11438158. https://pubmed.ncbi.nlm.nih.gov/11438158/. Retrieved December 4, 2023.
- ↑ "Raynaud's phenomenon". Joint Bone Spine (Elsevier BV) 74 (1): e1–e8. January 2007. doi:10.1016/j.jbspin.2006.07.002. PMID 17218139. https://pubmed.ncbi.nlm.nih.gov/17218139/. Retrieved December 4, 2023.
- ↑ "Bilateral, asymptomatic scaly and fissured cutaneous lesions of the fingers in a patient presenting with myositis". Indian Journal of Dermatology, Venereology and Leprology (Scientific Scholar) 71 (2): 137–138. 2005. doi:10.4103/0378-6323.14009. PMID 16394397.
- ↑ "The clinical phenotype associated with myositis-specific and associated autoantibodies: a meta-analysis revisiting the so-called antisynthetase syndrome". Autoimmunity Reviews (Elsevier BV) 13 (9): 883–891. September 2014. doi:10.1016/j.autrev.2014.03.004. PMID 24704867. https://pubmed.ncbi.nlm.nih.gov/24704867/. Retrieved December 4, 2023.
- ↑ "Evolution of Pulmonary Function in a Cohort of Patients with Interstitial Lung Disease and Positive for Antisynthetase Antibodies". The Journal of Rheumatology 47 (3): 415–423. March 2020. doi:10.3899/jrheum.181141. PMID 31203227. https://pubmed.ncbi.nlm.nih.gov/31203227/. Retrieved December 4, 2023.
- ↑ "Clinical features and outcomes of the patients with anti-glycyl tRNA synthetase syndrome". Clinical Rheumatology (Springer Science and Business Media LLC) 39 (8): 2417–2424. August 2020. doi:10.1007/s10067-020-04979-8. PMID 32144624. https://pubmed.ncbi.nlm.nih.gov/32144624/. Retrieved December 4, 2023.
- ↑ 29.0 29.1 "Pulmonary hypertension in antisynthetase syndrome: prevalence, aetiology and survival". The European Respiratory Journal (European Respiratory Society (ERS)) 42 (5): 1271–1282. November 2013. doi:10.1183/09031936.00156312. PMID 23397301.
- ↑ "Myocarditis in Patients With Antisynthetase Syndrome: Prevalence, Presentation, and Outcomes". Medicine (Ovid Technologies (Wolters Kluwer Health)) 94 (26): e798. July 2015. doi:10.1097/md.0000000000000798. PMID 26131832.
- ↑ "Die Behandlung des M. Menière mit Betahistin: Kritische Anmerkungen zur BEMED-Studie" (in de). Laryngo- Rhino- Otologie (Georg Thieme Verlag KG) 96 (8): 519–521. August 2017. doi:10.1055/s-0043-113690. PMID 28850992.
- ↑ "Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system". Arthritis and Rheumatism 23 (8): 881–888. August 1980. doi:10.1002/art.1780230802. PMID 7406938. https://pubmed.ncbi.nlm.nih.gov/7406938/.
- ↑ Lazarou, Ilias N.; Guerne, Pierre-André (March 15, 2013). "Classification, Diagnosis, and Management of Idiopathic Inflammatory Myopathies". The Journal of Rheumatology 40 (5): 550–564. doi:10.3899/jrheum.120682. ISSN 0315-162X. PMID 23504386. https://www.jrheum.org/content/40/5/550.
- ↑ "Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years?". Chest 138 (6): 1464–1474. December 2010. doi:10.1378/chest.10-0180. PMID 21138882. https://pubmed.ncbi.nlm.nih.gov/21138882/.
- ↑ "The use of auto-antibody testing in the evaluation of interstitial lung disease (ILD)--A practical approach for the pulmonologist". Respiratory Medicine 113: 80–92. April 2016. doi:10.1016/j.rmed.2016.01.019. PMID 26921132.
- ↑ "Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis". Arthritis Care & Research 65 (8): 1316–1324. August 2013. doi:10.1002/acr.21985. PMID 23908005.
- ↑ 37.0 37.1 "Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies". Rheumatic Disease Clinics of North America 28 (4): 859–90, viii. November 2002. doi:10.1016/s0889-857x(02)00032-7. PMID 12506776. https://pubmed.ncbi.nlm.nih.gov/12506776/. Retrieved December 4, 2023.
- ↑ "In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease". Autoimmunity 39 (3): 249–253. May 2006. doi:10.1080/08916930600623791. PMID 16769659. https://pubmed.ncbi.nlm.nih.gov/16769659/. Retrieved December 4, 2023.
- ↑ "Clinical characteristics of patients with anti-Jo-1 antibodies: a single center experience". Journal of Clinical Rheumatology 15 (5): 254–255. August 2009. doi:10.1097/RHU.0b013e3181b0e910. PMID 19590436.
- ↑ "Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings". European Journal of Radiology 84 (3): 516–523. March 2015. doi:10.1016/j.ejrad.2014.11.026. PMID 25541020. https://pubmed.ncbi.nlm.nih.gov/25541020/. Retrieved December 4, 2023.
- ↑ "An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias". American Journal of Respiratory and Critical Care Medicine 188 (6): 733–748. September 2013. doi:10.1164/rccm.201308-1483ST. PMID 24032382.
- ↑ "The pulmonary histopathologic manifestations of the anti-Jo-1 tRNA synthetase syndrome". Modern Pathology 23 (6): 874–880. June 2010. doi:10.1038/modpathol.2010.65. PMID 20228783.
- ↑ "Muscle biopsy findings in inflammatory myopathies". Rheumatic Disease Clinics of North America 28 (4): 779–98, vi. November 2002. doi:10.1016/s0889-857x(02)00030-3. PMID 12506772. https://pubmed.ncbi.nlm.nih.gov/12506772/. Retrieved December 4, 2023.
- ↑ "Drug therapy of the idiopathic inflammatory myopathies: predictors of response to prednisone, azathioprine, and methotrexate and a comparison of their efficacy". The American Journal of Medicine 94 (4): 379–387. April 1993. doi:10.1016/0002-9343(93)90148-i. PMID 8386437. https://pubmed.ncbi.nlm.nih.gov/8386437/. Retrieved December 4, 2023.
- ↑ "Azathioprine with prednisone for polymyositis. A controlled, clinical trial". Annals of Internal Medicine 92 (3): 365–369. March 1980. doi:10.7326/0003-4819-92-3-365. PMID 6986827. https://pubmed.ncbi.nlm.nih.gov/6986827/. Retrieved December 4, 2023.
- ↑ "Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease". The Journal of Rheumatology 40 (5): 640–646. May 2013. doi:10.3899/jrheum.121043. PMID 23457378.
- ↑ "Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease". The Journal of Rheumatology 40 (4): 484–492. April 2013. doi:10.3899/jrheum.121026. PMID 23418387. https://pubmed.ncbi.nlm.nih.gov/23418387/. Retrieved December 4, 2023.
- ↑ "Tacrolimus in refractory polymyositis with interstitial lung disease". Lancet 353 (9166): 1762–1763. May 1999. doi:10.1016/S0140-6736(99)01927-3. PMID 10347992.
- ↑ "Effects of tacrolimus on dermatomyositis and polymyositis: a prospective, open, non-randomized study of nine patients and a review of the literature". Clinical Rheumatology 31 (10): 1493–1498. October 2012. doi:10.1007/s10067-012-2044-y. PMID 22886003. https://pubmed.ncbi.nlm.nih.gov/22886003/. Retrieved December 4, 2023.
- ↑ "Benefit of adjunctive tacrolimus in connective tissue disease-interstitial lung disease". Pulmonary Pharmacology & Therapeutics 36: 46–52. February 2016. doi:10.1016/j.pupt.2015.12.004. PMID 26762710.
- ↑ "Treatment of antisynthetase-associated interstitial lung disease with tacrolimus". Arthritis and Rheumatism 52 (8): 2439–2446. August 2005. doi:10.1002/art.21240. PMID 16052580.
- ↑ "Rituximab treatment of the anti-synthetase syndrome: a retrospective case series". Rheumatology 48 (8): 968–971. August 2009. doi:10.1093/rheumatology/kep157. PMID 19531628. https://pubmed.ncbi.nlm.nih.gov/19531628. Retrieved December 4, 2023.
- ↑ "Severe interstitial lung disease in connective tissue disease: rituximab as rescue therapy". The European Respiratory Journal 40 (3): 641–648. September 2012. doi:10.1183/09031936.00163911. PMID 22282550.
- ↑ "Cyclophosphamide treatment for idiopathic inflammatory myopathies and related interstitial lung disease: a systematic review". Clinical Rheumatology 34 (1): 99–105. January 2015. doi:10.1007/s10067-014-2803-z. PMID 25367345. https://pubmed.ncbi.nlm.nih.gov/25367345/.
- ↑ "A retrospective review of clinical features and treatment outcomes in steroid-resistant interstitial lung disease from polymyositis/dermatomyositis". Respiratory Medicine 107 (6): 890–896. June 2013. doi:10.1016/j.rmed.2013.02.015. PMID 23517887. https://www.resmedjournal.com/article/S0954-6111(13)00064-4/fulltext.
- ↑ "A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis". The New England Journal of Medicine 329 (27): 1993–2000. December 1993. doi:10.1056/NEJM199312303292704. PMID 8247075.
- ↑ "Polymyositis associated with severe interstitial lung disease: remission after three doses of IV immunoglobulin". Chest 139 (2): 441–443. February 2011. doi:10.1378/chest.10-0360. PMID 21285059. https://pubmed.ncbi.nlm.nih.gov/21285059. Retrieved December 4, 2023.
- ↑ "CD19-targeted CAR T cells in refractory antisynthetase syndrome". Lancet (Elsevier) 401 (10379): 815–818. March 2023. doi:10.1016/S0140-6736(23)00023-5. PMID 36930673. https://pubmed.ncbi.nlm.nih.gov/36930673. Retrieved December 4, 2023.
- ↑ "CD19-Targeting CAR T-Cell Therapy for Antisynthetase Syndrome". JAMA 329 (24): 2130–2131. June 2023. doi:10.1001/jama.2023.7240. PMID 37367988. https://pubmed.ncbi.nlm.nih.gov/37367988. Retrieved December 4, 2023.
- ↑ 60.0 60.1 "Functional outcome and prognostic factors in anti-Jo1 patients with antisynthetase syndrome". Arthritis Research & Therapy 15 (5): R149. October 2013. doi:10.1186/ar4332. PMID 24286268.
- ↑ "Bi-lung transplantation in anti-synthetase syndrome with life-threatening interstitial lung disease". Rheumatology 57 (9): 1688–1689. September 2018. doi:10.1093/rheumatology/key123. PMID 29718458. https://pubmed.ncbi.nlm.nih.gov/29718458. Retrieved December 4, 2023.
- ↑ "Physical exercise as a treatment for adult and juvenile myositis". Journal of Internal Medicine 280 (1): 75–96. July 2016. doi:10.1111/joim.12481. PMID 26854121.
- ↑ 63.0 63.1 63.2 Rojas-Serrano, Jorge; Herrera-Bringas, Denisse; Mejía, Mayra; Rivero, Hermes; Mateos-Toledo, Heidegger; Figueroa, José E. (2015). "Prognostic factors in a cohort of antisynthetase syndrome (ASS): serologic profile is associated with mortality in patients with interstitial lung disease (ILD)". Clinical Rheumatology 34 (9): 1563–1569. doi:10.1007/s10067-015-3023-x. ISSN 0770-3198. PMID 26219488. https://link.springer.com/article/10.1007/s10067-015-3023-x. Retrieved December 4, 2023.
- ↑ Marie, I.; Josse, S.; Decaux, O.; Dominique, S.; Diot, E.; Landron, C.; Roblot, P.; Jouneau, S. et al. (2012). "Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome". Autoimmunity Reviews (Elsevier BV) 11 (10): 739–745. doi:10.1016/j.autrev.2012.01.006. ISSN 1568-9972. PMID 22326685. https://www.sciencedirect.com/science/article/abs/pii/S1568997212000201. Retrieved December 4, 2023.
- ↑ Rozelle, Andrew; Trieu, Sandy; Chung, Lorinda (2008). "Malignancy in the Setting of the Anti-Synthetase Syndrome". JCR: Journal of Clinical Rheumatology (Ovid Technologies (Wolters Kluwer Health)) 14 (5): 285–288. doi:10.1097/rhu.0b013e31817d116f. ISSN 1076-1608. PMID 18664993. https://pubmed.ncbi.nlm.nih.gov/18664993/. Retrieved December 4, 2023.
- ↑ Legault, Dominic; McDermott, John; Crous-Tsanaclis, Ana Maria; Boire, Gilles (January 1, 2008). "Cancer-associated myositis in the presence of anti-Jo1 autoantibodies and the antisynthetase syndrome.". The Journal of Rheumatology 35 (1): 169–171. ISSN 0315-162X. PMID 18176990. https://www.jrheum.org/content/35/1/169.long. Retrieved December 4, 2023.
- ↑ Castañeda-Pomeda, Marta; Prieto-González, Sergio; Grau, Josep Maria (2011). "Antisynthetase Syndrome and Malignancy". JCR: Journal of Clinical Rheumatology (Ovid Technologies (Wolters Kluwer Health)) 17 (8): 458. doi:10.1097/rhu.0b013e31823b1878. ISSN 1076-1608. PMID 22126809. https://pubmed.ncbi.nlm.nih.gov/22126809/. Retrieved December 4, 2023.
- ↑ Zang, Yuan-Sheng; Xiu, Qing-Yu; Fang, Zheng; Li, Bing; Xia, Tian-Bao (January 1, 2008). "Case Report: Dramatic Recovery of Lung Adenocarcinoma–Associated Dermatomyositis with Targeted Lung Cancer Therapy Alone". The Oncologist (Oxford University Press (OUP)) 13 (1): 79–81. doi:10.1634/theoncologist.2007-0172. ISSN 1083-7159. PMID 18245014.
- ↑ Noguchi, Eri; Uruha, Akinori; Suzuki, Shigeaki; Hamanaka, Kohei; Ohnuki, Yuko; Tsugawa, Jun; Watanabe, Yurika; Nakahara, Jin et al. (August 1, 2017). "Skeletal Muscle Involvement in Antisynthetase Syndrome". JAMA Neurology (American Medical Association (AMA)) 74 (8): 992–999. doi:10.1001/jamaneurol.2017.0934. ISSN 2168-6149. PMID 28586844.
- ↑ Ghirardello, A.; Zampieri, S.; Tarricone, E.; Iaccarino, L.; Bendo, R.; Briani, C.; Rondinone, R.; Sarzi-Puttini, P. et al. (2006). "Clinical implications of autoantibody screening in patients with autoimmune myositis". Autoimmunity 39 (3): 217–221. doi:10.1080/08916930600622645. ISSN 0891-6934. PMID 16769655. https://www.tandfonline.com/doi/full/10.1080/08916930600622645. Retrieved December 4, 2023.
Further reading
- "Antisynthetase syndrome pathogenesis: knowledge and uncertainties". Current Opinion in Rheumatology (Ovid Technologies (Wolters Kluwer Health)) 30 (6): 664–673. November 2018. doi:10.1097/bor.0000000000000555. PMID 30239350. https://pubmed.ncbi.nlm.nih.gov/30239350.
- Pinal-Fernandez, Iago; Casal-Dominguez, Maria; Huapaya, Julio A.; Albayda, Jemima; Paik, Julie J.; Johnson, Cheilonda; Silhan, Leann; Christopher-Stine, Lisa et al. (March 4, 2017). "A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies". Rheumatology (Oxford University Press (OUP)) 56 (6): 999–1007. doi:10.1093/rheumatology/kex021. ISSN 1462-0324. PMID 28339994.
- Koenig, Martial; Fritzler, Marvin J; Targoff, Ira N; Troyanov, Yves; Senécal, Jean-Luc (2007). "Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes". Arthritis Research & Therapy 9 (4): R78. doi:10.1186/ar2276. PMID 17688695.
External links
| Classification | |
|---|---|
| External resources |
 |

