Medicine:Interstitial lung disease
| Interstitial lung disease | |
|---|---|
| Other names | Diffuse parenchymal lung disease (DPLD) |
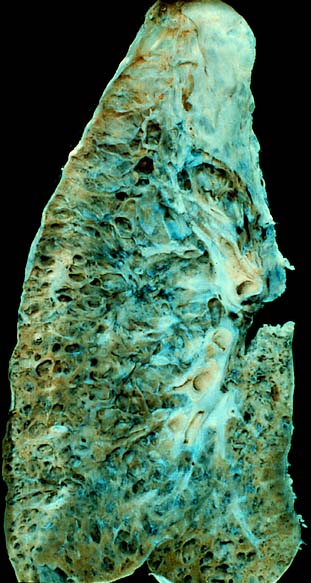 | |
| End-stage pulmonary fibrosis of unknown origin, taken from an autopsy | |
| Specialty | Pulmonology |
| Complications | Respiratory failure |
| Usual onset | Months – years, depending on cause/type |
| Duration | Months – years, depending on cause/type |
| Frequency | 1.9 million (2015)[1] |
| Deaths | 122,000 (2015)[2] |
Interstitial lung disease (ILD), or diffuse parenchymal lung disease (DPLD),[3] is a group of respiratory diseases affecting the interstitium (the tissue) and space around the alveoli (air sacs) of the lungs.[4] It concerns alveolar epithelium, pulmonary capillary endothelium, basement membrane, and perivascular and perilymphatic tissues. It may occur when an injury to the lungs triggers an abnormal healing response. Ordinarily, the body generates just the right amount of tissue to repair damage, but in interstitial lung disease, the repair process is disrupted, and the tissue around the air sacs (alveoli) becomes scarred and thickened. This makes it more difficult for oxygen to pass into the bloodstream. The disease presents itself with the following symptoms: shortness of breath, nonproductive coughing, fatigue, and weight loss, which tend to develop slowly, over several months. The average rate of survival for someone with this disease is between three and five years.[5] The term ILD is used to distinguish these diseases from obstructive airways diseases.
There are specific types in children, known as children's interstitial lung diseases. The acronym ChILD is sometimes used for this group of diseases.[6]
Prolonged ILD may result in pulmonary fibrosis, but this is not always the case. Idiopathic pulmonary fibrosis is interstitial lung disease for which no obvious cause can be identified (idiopathic) and is associated with typical findings both radiographic (basal and pleural-based fibrosis with honeycombing) and pathologic (temporally and spatially heterogeneous fibrosis, histopathologic honeycombing, and fibroblastic foci).
In 2015, interstitial lung disease, together with pulmonary sarcoidosis, affected 1.9 million people.[1] They resulted in 122,000 deaths.[2]
Causes

An ILD may be classified as to whether its cause is not known (idiopathic) or known (secondary).[7]
Idiopathic
Idiopathic interstitial pneumonia is the term given to ILDs with an unknown cause. They represent the majority of cases of interstitial lung diseases (up to two-thirds of cases).[8] They were subclassified by the American Thoracic Society in 2002 into 7 subgroups:[9]
- Idiopathic pulmonary fibrosis (IPF): the most common subgroup
- Desquamative interstitial pneumonia (DIP)
- Acute interstitial pneumonia (AIP): also known as Hamman-Rich syndrome
- Nonspecific interstitial pneumonia (NSIP)
- Respiratory bronchiolitis-associated interstitial lung disease (RB-ILD)
- Cryptogenic organizing pneumonia (COP): also known by the older name bronchiolitis obliterans organizing pneumonia (BOOP)
- Lymphoid interstitial pneumonia (LIP)
Secondary
Secondary ILDs are those diseases with a known etiology, including:[citation needed]
Connective tissue and autoimmune diseases
- Sarcoidosis
- Rheumatoid arthritis
- Systemic lupus erythematosus
- Systemic sclerosis
- Polymyositis
- Dermatomyositis
- Antisynthetase syndrome
Inhaled substances (pneumoconiosis)
- Inorganic
- Silicosis
- Asbestosis
- Berylliosis
- Industrial printing chemicals (e.g. carbon black, ink mist)
- Organic
- Hypersensitivity pneumonitis (extrinsic allergic alveolitis)
Drug-induced
- Antibiotics (e.g., nitrofurantoin and sulfa drugs)
- Chemotherapeutic drugs
- Antiarrhythmic agents
- Cigarette smoking[10]
- Smoking-related interstitial fibrosis (SRIF) is an example of a type of interstitial lung disease known to be caused by smoking.
Infection
- Coronavirus disease 2019 (COVID-19)[11]
- Atypical pneumonia
- Pneumocystis pneumonia (PCP)
- Tuberculosis
- Chlamydia trachomatis
- Respiratory syncytial virus
Malignancy
Childhood interstitial lung disease and ILD predominately in children
- Diffuse developmental disorders
- Growth abnormalities and deficient alveolarisation
- Infant conditions of undefined cause
- ILD related to alveolar surfactant region
Diagnosis
Investigation is tailored towards the symptoms and signs. A proper and detailed history looking for the occupational exposures and for signs of conditions listed above is the first and probably the most important part of the workup in patients with interstitial lung disease. Pulmonary function tests usually show a restrictive defect with decreased diffusion capacity (DLCO).[citation needed]
A lung biopsy is required if the clinical history and imaging are not clearly suggestive of a specific diagnosis or malignancy cannot otherwise be ruled out. In cases where a lung biopsy is indicated, a trans-bronchial biopsy is usually unhelpful, and a surgical lung biopsy is often required.[citation needed][9]
Pulmonary function testing
Most patients with suspected ILD are likely to undergo complete pulmonary function testing. These tests are useful in diagnosis and determining severity of the disease.[12]
Although there is large diversity in interstitial lung disease, most follow a restrictive pattern. Restrictive defects are defined by decreased TLC (total lung capacity), RV (residual volume), FVC (forced vital capacity) and FEV1 (forced expiratory volume in one second). As both FVC and FEV1 are reduced, the FVC to FEV1 ratio remains normal or is increased.[12]
As disease progression increases and the lungs become stiffer lung volumes will continue to decrease; lower TLC, RV, FVC and FEV1 scores are associated with a more severe disease progression and poorer prognosis.[12]
X-ray and CT (radiography and computed tomography)
Chest radiography is usually the first test to detect interstitial lung diseases, but the chest radiograph can be normal in up to 10% of patients, especially early in the disease process.[13][14]
High-resolution CT of the chest is the preferred modality and differs from routine CT of the chest. Conventional (regular) CT chest examines 7–10 mm slices obtained at 10 mm intervals; high resolution CT examines 1–1.5 mm slices at 10 mm intervals using a high-spatial-frequency reconstruction algorithm. The HRCT therefore provides approximately 10 times more resolution than the conventional CT chest, allowing the HRCT to elicit details that cannot otherwise be visualized.[13][15]
Radiologic appearance alone, however, is not adequate and should be interpreted in the clinical context, keeping in mind the temporal profile of the disease process.[13]
Interstitial lung diseases can be classified according to radiologic patterns.[13]
Pattern of opacities
- Consolidation
- Acute:
- Alveolar hemorrhage syndromes
- Acute eosinophilic pneumonia
- Acute interstitial pneumonia
- Cryptogenic organizing pneumonia
- Chronic:
- Chronic eosinophilic pneumonia
- Cryptogenic organizing pneumonia
- Lymphoproliferative disorders
- Pulmonary alveolar proteinosis
- Sarcoidosis
- Linear or reticular opacities
- Acute:
- Pulmonary edema
- Chronic:
- Idiopathic pulmonary fibrosis
- Connective tissue-associated interstitial lung diseases
- Asbestosis
- Sarcoidosis
- Hypersensitivity pneumonitis
- Drug-induced lung disease
- Small nodules
- Acute:
- Hypersensitivity pneumonitis
- Chronic:
- Hypersensitivity pneumonitis
- Sarcoidosis
- Silicosis
- Coal workers pneumoconiosis
- Respiratory bronchiolitis
- Alveolar microlithiasis
- Cystic airspaces
- Chronic:
- Pulmonary Langerhans cell histiocytosis
- Pulmonary lymphangioleiomyomatosis
- Honeycomb lung caused by idiopathic pulmonary fibrosis (IPF) or other diseases
- Ground glass opacities
- Acute:
- Alveolar hemorrhage syndromes
- Pulmonary edema
- Hypersensitivity pneumonitis
- Acute inhalational exposures
- Drug-induced lung diseases
- Acute interstitial pneumonia
- Chronic:
- Nonspecific interstitial pneumonia
- Respiratory bronchiolitis-associated interstitial lung disease
- Desquamative interstitial pneumonia
- Drug-induced lung diseases
- Pulmonary alveolar proteinosis
- Thickened alveolar septa
- Acute:
- Pulmonary edema
- Chronic:
- Lymphangitic carcinomatosis
- Pulmonary alveolar proteinosis
- Sarcoidosis
- Pulmonary veno-occlusive disease[13]
Distribution
- Upper lung predominance
- Pulmonary Langerhans cell histiocytosis
- Silicosis
- Coal workers pneumoconiosis
- Carmustine-related pulmonary fibrosis
- Respiratory broncholitis associated with interstitial lung disease
- Lower lung predominance
- Idiopathic pulmonary fibrosis
- Pulmonary fibrosis associated with connective tissue diseases (ILD-CTD)
- Asbestosis
- Chronic aspiration
- Central predominance (perihilar)
- Sarcoidosis
- Berylliosis
- Peripheral predominance
- Idiopathic pulmonary fibrosis
- Chronic eosinophilic pneumonia
- Cryptogenic organizing pneumonia[13]
Associated findings
- Pleural effusion or thickening
- Pulmonary edema
- Connective tissue diseases
- Asbestosis
- Lymphangitic carcinomatosis
- Lymphoma
- Lymphangioleiomyomatosis
- Drug-induced lung diseases
- Lymphadenopathy
- Sarcoidosis
- Silicosis
- Berylliosis
- Lymphangitic carcinomatosis
- Lymphoma
- Lymphocytic interstitial pneumonia[13]
Genetic testing
For some types of paediatric ILDs and few forms adult ILDs, genetic causes have been identified. These may be identified by blood tests. For a limited number of cases, this is a definite advantage, as a precise molecular diagnosis can be done; frequently then there is no need for a lung biopsy. Testing is available for
- Surfactant protein B deficiency (mutations in SFTPB)
- Surfactant protein C deficiency (mutations in SFTPC)
- ABCA3 deficiency (mutations in ABCA3)
- Brain–lung–thyroid syndrome (Mutations in TTF1)
- Congenital pulmonary alveolar proteinosis (mutations in CSFR2A and/or CSFR2B)
Diffuse developmental disorder
- Alveolar capillary dysplasia (mutations in FoxF1)
Idiopathic pulmonary fibrosis
- Mutations in telomerase reverse transcriptase (TERT)
- Mutations in telomerase RNA component (TERC)
- Mutations in the regulator of telomere elongation helicase 1 (RTEL1)
- Mutations in poly(A)-specific ribonuclease (PARN)
Treatment
ILD is not a single disease but encompasses many different pathological processes, hence treatment is different for each disease. If a specific occupational exposure cause is found, the person should avoid that environment. If a drug cause is suspected, that drug should be discontinued.[citation needed]
Many cases due to unknown or connective tissue-based causes are treated with corticosteroids,[16] such as prednisolone. Some people respond to immunosuppressant treatment.
Oxygen therapy
Oxygen therapy at home is recommended in those with significantly low oxygen levels.[17]
Pulmonary rehabilitation
Pulmonary rehabilitation appears to be useful with the benefits being sustainable longer term with improvement in exercise capacity, dyspnoea, and quality of life.[18] Lung transplantation is an option if the ILD progresses despite therapy in appropriately selected patients with no other contraindications.[19][20]
Antibodies and inhibitors
On October 16, 2014, the Food and Drug Administration approved a new drug for the treatment of idiopathic pulmonary fibrosis (IPF). This drug, Ofev (nintedanib), is marketed by Boehringer Ingelheim Pharmaceuticals, Inc. This drug has been shown to slow the decline of lung function, although the drug has not been shown to reduce mortality or improve lung function. The estimated cost of the drug per year is approximately $94,000.[21]
References
- ↑ 1.0 1.1 ((GBD 2015 Disease and Injury Incidence and Prevalence Collaborators)) (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMID 27733282.
- ↑ 2.0 2.1 ((GBD 2015 Mortality and Causes of Death Collaborators)) (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMID 27733281.
- ↑ "Clinical advances in the diagnosis and therapy of the interstitial lung diseases". American Journal of Respiratory and Critical Care Medicine 172 (3): 268–79. August 2005. doi:10.1164/rccm.200503-483OE. PMID 15879420.
- ↑ "Frequently Asked Questions About Interstitial Lung Disease". University of Chicago Medical Center. http://www.uchospitals.edu/specialties/pulmonary/interstitial-lung/faq.html.
- ↑ Meyer, Keith C; Decker, Catherine A (2017-04-03). "Role of pirfenidone in the management of pulmonary fibrosis". Therapeutics and Clinical Risk Management 13: 427–437. doi:10.2147/TCRM.S81141. ISSN 1176-6336. PMID 28435277.
- ↑ "European protocols for the diagnosis and initial treatment of interstitial lung disease in children". Thorax 70 (11): 1078–84. November 2015. doi:10.1136/thoraxjnl-2015-207349. PMID 26135832.
- ↑ "Interstitial lung disease: progress and problems". Postgraduate Medical Journal 82 (970): 494–9. August 2006. doi:10.1136/pgmj.2006.046417. PMID 16891438.
- ↑ Kreuter, Michael; Herth, Felix J. F.; Wacker, Margarethe; Leidl, Reiner; Hellmann, Andreas; Pfeifer, Michael; Behr, Jürgen; Witt, Sabine et al. (2015). "Exploring Clinical and Epidemiological Characteristics of Interstitial Lung Diseases: Rationale, Aims, and Design of a Nationwide Prospective Registry—The EXCITING-ILD Registry" (in en). BioMed Research International 2015: 123876. doi:10.1155/2015/123876. PMID 26640781.
- ↑ 9.0 9.1 "Interstitial Lung Diseases". 23 September 2020. https://www.lecturio.com/concepts/interstitial-lung-diseases/.
- ↑ "Smoking-related interstitial fibrosis (SRIF) in patients presenting with diffuse parenchymal lung disease". American Journal of Clinical Pathology 159 (2): 146–157. Dec 2022. doi:10.1093/ajcp/aqac144. PMID 36495281.
- ↑ "Dr. Daniele Macchini, Treating COVID-19 Patients in Bergamo, Italy, Describes Horrible Situation". 11 March 2020. https://oaklandnewsnow.com/2020/03/11/dr-daniele-macchini-treating-covid-19-patients-in-bergamo-italy-describes-horrible-situation/.
- ↑ 12.0 12.1 12.2 King, Talmadge E Jr (February 27, 2023). "Approach to the adult with interstitial lung disease: Diagnostic testing". https://www.uptodate.com/contents/approach-to-the-adult-with-interstitial-lung-disease-diagnostic-testing?search=ILD&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2#H10.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 "Diagnostic approach to the patient with diffuse lung disease". Mayo Clinic Proceedings 77 (11): 1221–7; quiz 1227. November 2002. doi:10.4065/77.11.1221. PMID 12440558.
- ↑ "What Is Pulmonary Fibrosis?". Northwestern Medicine. http://fibrosis.northwesternmedicine.org/frequently-asked-questions.html.
- ↑ "Radio-pathological correlation of organizing pneumonia (OP): a pictorial review". The British Journal of Radiology 90 (1071): 20160723. March 2017. doi:10.1259/bjr.20160723. PMID 28106480.
- ↑ "Interstitial lung disease - Diagnosis and Treatment". MayoClinic.com. April 25, 2023. https://www.mayoclinic.org/diseases-conditions/interstitial-lung-disease/diagnosis-treatment/drc-20353113.
- ↑ Hayes D, Jr; Wilson, KC; Krivchenia, K; Hawkins, SMM; Balfour-Lynn, IM; Gozal, D; Panitch, HB; Splaingard, ML et al. (1 February 2019). "Home Oxygen Therapy for Children. An Official American Thoracic Society Clinical Practice Guideline.". American Journal of Respiratory and Critical Care Medicine 199 (3): e5–e23. doi:10.1164/rccm.201812-2276ST. PMID 30707039.
- ↑ Dowman, Leona; Hill, Catherine J.; May, Anthony; Holland, Anne E. (2021-02-01). "Pulmonary rehabilitation for interstitial lung disease". The Cochrane Database of Systematic Reviews 2021 (2): CD006322. doi:10.1002/14651858.CD006322.pub4. ISSN 1469-493X. PMID 34559419.
- ↑ "Lung transplantation". American Journal of Respiratory and Critical Care Medicine 184 (2): 159–71. July 2011. doi:10.1164/rccm.201101-0134CI. PMID 21471083.
- ↑ "Lung transplantation for interstitial lung disease". Clinics in Chest Medicine 33 (1): 179–89. March 2012. doi:10.1016/j.ccm.2011.12.003. PMID 22365254.
- ↑ "FDA approves Ofev to treat idiopathic pulmonary fibrosis". U.S. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm418994.htm.
External links
| Classification | |
|---|---|
| External resources |
 |





