Medicine:Autoimmune encephalitis
| Autoimmune encephalitis | |
|---|---|
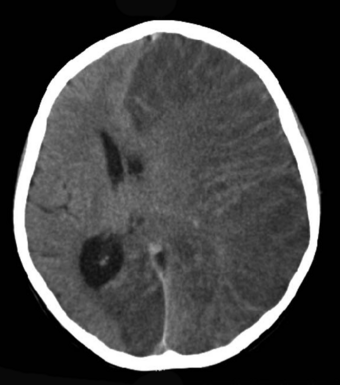 | |
| Brain CT scan without contrast enhancement of a patient, female, 8 years old, with Rasmussen's encephalitis. |
Autoimmune encephalitis (AIE) is a type of encephalitis, and one of the most common causes of noninfectious encephalitis. It can be triggered by tumors, infections, or it may be cryptogenic. The neurological manifestations can be either acute or subacute and usually develop within six weeks. The clinical manifestations include behavioral and psychiatric symptoms, autonomic disturbances, movement disorders, and seizures.[1]
Autoimmune encephalitis can result from a number of autoimmune diseases including:
- Rasmussen encephalitis
- Systemic lupus erythematosus
- Behçet's disease
- Hashimoto's encephalopathy
- Autoimmune limbic encephalitis[2]
- Sydenham's chorea
The severity of the condition can be monitored using the Modified Rankin Scale and the clinical assessment scale in autoimmune encephalitis (CASE) score.[3][4]
Signs and symptoms
Patients with AIE may present movement disorders such as ataxia, dystonia, myoclonus, and orofacial dyskinesia. Seizures are the most common symptom and different types of seizures may be seen, including refractory status epilepticus.[5] Autonomic disturbances such as sweating, hypertension, tachycardia and hypoventilation are also frequent. Some patients may develop gastrointestinal manifestations (diarrhea, gastroparesis, and constipation) due to involvement of the myenteric plexus. Sleep disturbances such as insomnia, abnormal sleep movements, sleep apnea, and hypersomnia are also found.[6][1]
Some of these findings are suggestive of certain types of encephalitis and may indicate a specific underlying antibody or tumor.[1]
Mechanism
Autoimmune encephalitis commonly presents an immune response against neuronal autoantigens with production of antibodies.[7] Anti-neuronal antibodies are classified into antibodies against cell surface antigens (CSAab), antibodies against synaptic antigens (SyAab) and antibodies against intraneuronal antigens (INAab), also known as onconeural antibodies.[7][1]
Diagnosis
Diagnostic criteria for possible autoimmune encephalitis (all three of the following criteria met):[1]
- Subacute onset (rapid progression of less than three months) of working memory deficits (short-term memory loss), altered mental status (decreased level of consciousness, lethargy or personality changes), or psychiatric symptoms
- At least one of the following:
- New focal central nervous system findings
- Seizures not explained by previously-known seizure disorder
- Cerebrospinal fluid pleocytosis
- Magnetic resonance imaging suggestive of encephalitis
- Seizures not explained by a previously known seizure disorder. In such cases, an electroencephalogram (EEG) can be a valuable tool to assess brain electrical activity and identify patterns consistent with seizure disorders or encephalopathy, further supporting the suspicion of autoimmune encephalitis.
- Reasonable exclusion of alternative causes
Criteria for autoantibody-negative but probable autoimmune encephalitis (all four criteria met):
- Subacute onset (rapid progression of less than three months) of working memory deficits (short-term memory loss), altered mental status (decreased level of consciousness, lethargy or personality changes), or psychiatric symptoms
- Exclusion of well-defined syndromes of autoimmune encephalitis (typical limbic encephalitis, Bickerstaff brainstem encephalitis, acute disseminated encephalomyelitis)
- Absence of well-characterized autoantibodies in blood serum and cerebrospinal fluid, and at least two of the following criteria:
- Magnetic resonance imaging suggestive of encephalitis
- CSF pleocytosis, oligoclonal bands or elevated cerebrospinal fluid IgG index, or both
- Brain biopsy showing inflammatory infiltrates and excluding other disorders
- Reasonable exclusion of alternative causes
Classification
Anti-NMDAR encephalitis
Anti-N-methyl-D-aspartate receptor encephalitis is one of the most common causes of AIE and was originally described in 2007 in a cohort of 12 patients, 11 of them with ovarian teratomas.[8] This condition predominantly affects children and young female patients.[9] Underlying malignancies are found mainly in patients between the age of 12–45 years; most of them are ovarian teratomas (94%), followed by extraovarian teratomas (2%), and other tumors (4%). Herpes simplex virus-1 encephalitis appears to be a trigger for anti-NMDAR encephalitis; most AIE cases after herpes zoster are now believed to be anti-NMDAR encephalitis.[10][1]
Anti-AMPAR encephalitis
Patients with anti-Α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (anti-AMPAR) encephalitis characteristically present with seizures, memory impairment and psychosis. Some may develop sleep disturbances and movement disorders. Anti-AMPAR encephalitis is paraneoplastic in etiology in 64% of cases, mostly associated with thymoma, ovarian teratoma and lung and breast cancer. Brain MRI shows T2 and FLAIR hyperintensities, particularly in the medial temporal lobe. Lesions in the brain cortex or subcortex, sometimes with demyelination, may also be found. Cerebrospinal fluid (CSF) examination may show pleocytosis and oligoclonal bands.[11][1]
Anti-GABA encephalitis
Anti-GABA-AR encephalitis
Anti-gamma-aminobutyric acid A receptor (anti-GABA-AR) encephalitis was first reported in 2014 in six patients (two male children, one female teenager and three male adults). They developed a rapidly progressive encephalopathy with early behavioral or cognitive changes that evolved with refractory seizures and multifocal lesions as seen on brain magnetic resonance imaging.[12] In most of these patients, CSF analysis showed lymphocytic pleocytosis. A recent study identified an underlying neoplasia in 27% of these patients, mostly thymomas.[13] Similar to that seen in patients with anti-gamma-aminobutyric acid B receptor (GABA-BR) and anti-AMPAR antibodies, they may also present with coexisting autoimmune disorders such as thyroiditis or myasthenia.[14][1]
Anti-GABA-BR encephalitis
Anti-GABA-BR encephalitis is characterized by cognitive symptoms with severe seizures or status epilepticus.[15] Other presentations include ataxia and opsoclonus-myoclonus. In a small series of 20 patients with anti-GABA-BR, about 50% were found to have small-cell lung cancer.[16] Males and females appear to be equally affected. The long-term prognosis in anti-GABA-BR encephalitis is determined by the presence of an underlying malignancy.[17][1]
Anti-LGI1 and anti-CASPR2 encephalitis
The first reports of anti-voltage-gated potassium channel-complex antibodies (anti-VGKC) date back to 2001 and described patients with neuromyotonia, Morvan's syndrome and limbic encephalitis.[18] Other rare phenotypes included epilepsy and painful polyneuropathy. Anti-VGKC antibodies, in fact, later turned out to be directed against proteins that form a complex with VGKC called leucine-rich glioma-inactivated 1 (LGI1) and contactin-associated protein-like 2 (CASPR-2).[19][20] Each of these antibodies lead to specific clinical symptoms.[1]
Anti-GAD encephalitis
Glutamic acid decarboxylase (GAD) is an enzyme that catalyzes the conversion of glutamic acid to the neurotransmitter GABA. Anti-GAD antibodies have been associated with other autoimmune disorders such as insulin-dependent diabetes mellitus. The main neurological syndromes associated with anti-GAD antibodies include stiff-person syndrome, cerebellar ataxia, epilepsy and limbic encephalitis.[21][1]
Anti-GlyR encephalitis
Glycine receptors (GlyR) are chloride channels that facilitate inhibitory neurotransmission in the brain and spinal cord. Anti-GlyR antibodies were first described in patients with progressive encephalomyelitis with rigidity and myoclonus and later in patients with stiff-person syndrome.[22][23] Recently, anti-GlyR antibodies have also been reported in patients with cerebellar ataxia and anti-GAD antibodies and patients with demyelinating diseases including optic neuritis and multiple sclerosis, but their clinical significance remains unclear.[24][25] Anti-GlyR antibodies are usually not associated with tumors, although there have been reports of patients with underlying thymoma, small-cell lung cancer, breast cancer and chronic lymphocytic leukemia.[1]
Anti-DPPX encephalitis
Dipeptidyl peptidase-like protein 6 (DPPX) is a subunit of Kv4.2 potassium channels expressed in the hippocampus, cerebellum, striatum, and myenteric plexus. Patients with anti-DPPX antibodies show neuropsychiatric symptoms (agitation and confusion), myoclonus, tremor, startle reflex, seizures, stiff-person syndrome and prodromal diarrhea of unknown etiology. In addition, they may have symptoms of dysautonomia including arrhythmias, thermodysregulation, diaphoresis, urinary symptoms and sleep disorders.[26][27][1]
Encephalopathy associated with anti-IgLON5 antibodies
The IgLON family member 5 (IgLON5) is a neuronal cell adhesion molecule of the immunoglobulin superfamily. Patients with anti-IgLON5 antibodies present with a unique non-REM (rapid eye movement) and REM parasomnia with obstructive sleep apnea, stridor, episodic central hypoventilation, dementia, gait instability, chorea, dysarthria, dysphagia, dysautonomia and supranuclear gaze palsy resembling that seen in classic tauopathy.[28][27] All published cases reported the presence of the alleles HLA-DQB1*0501 and HLA-DRB1*1001 suggesting genetic susceptibility to this disease. Neuropathological postmortem studies have shown a novel tauopathy with extensive neuronal deposits of hyperphosphorylated tau mainly involving the tegmentum of the brainstem and hypothalamus. This novel encephalopathy provides an intriguing link between neurodegeneration and cell-surface autoimmunity. A recent study has shown that anti-IgLON5 antibodies recognize Ig-like domain 2 as an immunogenic region and causes irreversible internalization of IgLON5 from the neuronal membrane. These findings support a potential pathogenic role of anti-IgLON5 antibodies in the associated encephalopathy.[29][1]
Anti-mGluR1 and anti-mGluR5 encephalitis
Metabotropic glutamate receptor 1 (mGluR1) and metabotropic glutamate receptor 5 (mGluR5) are both G-protein-coupled receptors that share an 85% amino acid sequence homology. Both receptors are involved in modulating synaptic functions including the electrical change in neuronal response called long-term depression (a term not related to the mood-changing disorder major depression). While mGluR1 facilitates long-term depression at parallel fiber to Purkinje cell synapses, which are critical for cerebellar motor learning, mGluR5 is more relevant for long-term depression in the hippocampus.[1]
All patients with anti-mGluR1 antibodies develop cerebellar ataxia of subacute onset, and some may present with additional symptoms such as paranoia, dysgeusia, diplopia and cognitive deficits. Common tumors found to be associated with anti-mGluR1 antibodies are hematologic malignancies and prostate adenocarcinoma.[30][1]
Patients with anti-mGluR5-abs present with a form of encephalitis named "Ophelia syndrome", a clinical syndrome that includes memory loss and psychosis in association with Hodgkin's lymphoma.[31] The outcome of reported cases is generally good after treatment of the lymphoma and immunotherapy.[31][1]
Seronegative autoimmune encephalitis
Autoimmune encephalitis might occur without the identification of any pathogenic antibody, in which case it is called seronegative autoimmune encephalitis.[4]
It can be further categorized in three subtypes: antibody-negative probable autoimmune encephalitis, autoimmune limbic encephalitis and acute disseminated encephalomyelitis.[4]
One therapeutic approach to seronegative autoimmune encephalitis is using as a first-line treatment corticosteroids and intravenous immunoglobulin.[4]Other options include the use of rituximab (second-line) and tocilizumab or cyclophosphamide (next-line).[4]
A study in a South Korean hospital with 142 patients identified 5 factors that should be considered when evaluating the disease:[4]
- Presence of refractory status epilepticus
- Advanced age of onset (over or equal to 60 years)
- Having the subtype of probable AE (ANPRA)
- Infra-tentorium involvement in brain magnetic resonance imaging
- Delay of immunotherapy of more than 1 month
The less of those factors are present, the better the chance of good recovery in a 2-year period.[4]
See also
- Encephalitis
- Viral encephalitis
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Dutra, Lívia Almeida; Abrantes, Fabiano; Toso, Fabio Fieni; Pedroso, José Luiz; Barsottini, Orlando Graziani Povoas; Hoftberger, Romana (January 2018). "Autoimmune encephalitis: a review of diagnosis and treatment" (in en). Arquivos de Neuro-Psiquiatria 76 (1): 41–49. doi:10.1590/0004-282X20170176. ISSN 0004-282X. PMID 29364393.
 This article incorporates text available under the CC BY 4.0 license.
This article incorporates text available under the CC BY 4.0 license.
- ↑ Bhalla, D.; Godet, B.; Druet-Cabanac, M.; Preux, PM. (Jun 2011). "Etiologies of epilepsy: a comprehensive review.". Expert Rev Neurother 11 (6): 861–76. doi:10.1586/ern.11.51. PMID 21651333.
- ↑ Lim, Jung-Ah; Lee, Soon-Tae; Moon, Jangsup; Jun, Jin-Sun; Kim, Tae-Joon; Shin, Yong-Won; Abdullah, Suhailah; Byun, Jung Ick et al. (10 February 2019). "Development of the clinical assessment scale in autoimmune encephalitis". Annals of Neurology 85 (3): 352–358. doi:10.1002/ANA.25421. PMID 30675918. https://www.wikidata.org/wiki/Q91191724.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 , Wikidata Q115688145
- ↑ Davis, Rebecca; Dalmau, Josep (September 2013). "Autoimmunity, seizures, and status epilepticus" (in en). Epilepsia 54 (6): 46–49. doi:10.1111/epi.12276. PMID 24001072.
- ↑ Tobin, William Oliver; Lennon, Vanda A.; Komorowski, Lars; Probst, Christian; Clardy, Stacey Lynn; Aksamit, Allen J.; Appendino, Juan Pablo; Lucchinetti, Claudia F. et al. (11 November 2014). "DPPX potassium channel antibody: Frequency, clinical accompaniments, and outcomes in 20 patients" (in en). Neurology 83 (20): 1797–1803. doi:10.1212/WNL.0000000000000991. ISSN 0028-3878. PMID 25320100. PMC 4240433. https://n.neurology.org/content/83/20/1797.
- ↑ 7.0 7.1 Lancaster, Eric; Dalmau, Josep (July 2012). "Neuronal autoantigens—pathogenesis, associated disorders and antibody testing" (in en). Nature Reviews Neurology 8 (7): 380–390. doi:10.1038/nrneurol.2012.99. ISSN 1759-4766. PMID 22710628.
- ↑ Dalmau, Josep; Tüzün, Erdem; Wu, Hai-yan; Masjuan, Jaime; Rossi, Jeffrey E.; Voloschin, Alfredo; Baehring, Joachim M.; Shimazaki, Haruo et al. (January 2007). "Paraneoplastic anti- N -methyl-D-aspartate receptor encephalitis associated with ovarian teratoma" (in en). Annals of Neurology 61 (1): 25–36. doi:10.1002/ana.21050. PMID 17262855.
- ↑ Graus, Francesc; Titulaer, Maarten J.; Balu, Ramani; Benseler, Susanne; Bien, Christian G.; Cellucci, Tania; Cortese, Irene; Dale, Russell C. et al. (1 April 2016). "A clinical approach to diagnosis of autoimmune encephalitis" (in English). The Lancet Neurology 15 (4): 391–404. doi:10.1016/S1474-4422(15)00401-9. ISSN 1474-4422. PMID 26906964.
- ↑ Graus, Francesc; Titulaer, Maarten J; Balu, Ramani; Benseler, Susanne; Bien, Christian G; Cellucci, Tania; Cortese, Irene; Dale, Russell C et al. (April 2016). "A clinical approach to diagnosis of autoimmune encephalitis" (in en). The Lancet Neurology 15 (4): 391–404. doi:10.1016/S1474-4422(15)00401-9. PMID 26906964.
- ↑ Höftberger, Romana; Sonderen, Agnes van; Leypoldt, Frank; Houghton, David; Geschwind, Michael; Gelfand, Jeffrey; Paredes, Mercedes; Sabater, Lidia et al. (16 June 2015). "Encephalitis and AMPA receptor antibodies: Novel findings in a case series of 22 patients" (in en). Neurology 84 (24): 2403–2412. doi:10.1212/WNL.0000000000001682. ISSN 0028-3878. PMID 25979696. PMC 4478035. https://n.neurology.org/content/84/24/2403.
- ↑ Petit-Pedrol, Mar; Armangue, Thaís; Peng, Xiaoyu; Bataller, Luis; Cellucci, Tania; Davis, Rebecca; McCracken, Lindsey; Martinez-Hernandez, Eugenia et al. (1 March 2014). "Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies" (in English). The Lancet Neurology 13 (3): 276–286. doi:10.1016/S1474-4422(13)70299-0. ISSN 1474-4422. PMID 24462240.
- ↑ Spatola, Marianna; Petit-Pedrol, Mar; Simabukuro, Mateus Mistieri; Armangue, Thaís; Castro, Fernanda J.; Artigues, Maria I. Barcelo; Benique, Maria R. Julià; Benson, Leslie et al. (14 March 2017). "Investigations in GABAA receptor antibody-associated encephalitis" (in en). Neurology 88 (11): 1012–1020. doi:10.1212/WNL.0000000000003713. ISSN 0028-3878. PMID 28202703. PMC 5384834. https://n.neurology.org/content/88/11/1012.
- ↑ Ohkawa, Toshika; Satake, Shin'Ichiro; Yokoi, Norihiko; Miyazaki, Yu; Ohshita, Tomohiko; Sobue, Gen; Takashima, Hiroshi; Watanabe, Osamu et al. (11 June 2014). "Identification and Characterization of GABAA Receptor Autoantibodies in Autoimmune Encephalitis" (in en). Journal of Neuroscience 34 (24): 8151–8163. doi:10.1523/JNEUROSCI.4415-13.2014. ISSN 0270-6474. PMID 24920620. PMC 6608235. https://www.jneurosci.org/content/34/24/8151.
- ↑ Lancaster, Eric; Lai, Meizan; Peng, Xiaoyu; Hughes, Ethan; Constantinescu, Radu; Raizer, Jeffrey; Friedman, Daniel; Skeen, Mark B. et al. (1 January 2010). "Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen" (in English). The Lancet Neurology 9 (1): 67–76. doi:10.1016/S1474-4422(09)70324-2. ISSN 1474-4422. PMID 19962348.
- ↑ Höftberger, Romana; Titulaer, Maarten J.; Sabater, Lidia; Dome, Balazs; Rózsás, Anita; Hegedus, Balazs; Hoda, Mir Alireza; Laszlo, Viktoria et al. (22 October 2013). "Encephalitis and GABAB receptor antibodies: Novel findings in a new case series of 20 patients" (in en). Neurology 81 (17): 1500–1506. doi:10.1212/WNL.0b013e3182a9585f. ISSN 0028-3878. PMID 24068784. PMC 3888170. https://n.neurology.org/content/81/17/1500.
- ↑ Lancaster, Eric; Martinez-Hernandez, Eugenia; Dalmau, Josep (12 July 2011). "Encephalitis and antibodies to synaptic and neuronal cell surface proteins" (in en). Neurology 77 (2): 179–189. doi:10.1212/WNL.0b013e318224afde. ISSN 0028-3878. PMID 21747075. PMC 3140073. https://n.neurology.org/content/77/2/179.
- ↑ Sonderen, Agnes van; Schreurs, Marco W. J.; Bruijn, Marienke A. A. M. de; Boukhrissi, Sanae; Nagtzaam, Mariska M. P.; Hulsenboom, Esther S. P.; Enting, Roelien H.; Thijs, Roland D. et al. (3 May 2016). "The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies" (in en). Neurology 86 (18): 1692–1699. doi:10.1212/WNL.0000000000002637. ISSN 0028-3878. PMID 27037230. https://n.neurology.org/content/86/18/1692.
- ↑ Irani, Sarosh R.; Pettingill, Philippa; Kleopa, Kleopas A.; Schiza, Natasa; Waters, Patrick; Mazia, Claudio; Zuliani, Luigi; Watanabe, Osamu et al. (August 2012). "Morvan syndrome: Clinical and serological observations in 29 cases" (in en). Annals of Neurology 72 (2): 241–255. doi:10.1002/ana.23577. PMID 22473710. https://onlinelibrary.wiley.com/doi/10.1002/ana.23577.
- ↑ van Sonderen, A.; Schreurs, M. W. J.; Wirtz, P. W.; Sillevis Smitt, P. A. E.; Titulaer, M. J. (1 October 2016). "From VGKC to LGI1 and Caspr2 encephalitis: The evolution of a disease entity over time" (in en). Autoimmunity Reviews 15 (10): 970–974. doi:10.1016/j.autrev.2016.07.018. ISSN 1568-9972. PMID 27485013.
- ↑ Vale, Thiago Cardoso; Pedroso, José Luiz; Alquéres, Rafaela Almeida; Dutra, Lívia Almeida; Barsottini, Orlando Graziani Povoas (15 December 2015). "Spontaneous downbeat nystagmus as a clue for the diagnosis of ataxia associated with anti-GAD antibodies" (in English). Journal of the Neurological Sciences 359 (1): 21–23. doi:10.1016/j.jns.2015.10.024. ISSN 0022-510X. PMID 26671081. https://www.jns-journal.com/article/S0022-510X(15)02494-6/abstract.
- ↑ Alexopoulos, Harry; Akrivou, Sofia; Dalakas, Marinos C. (26 November 2013). "Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders" (in en). Neurology 81 (22): 1962–1964. doi:10.1212/01.wnl.0000436617.40779.65. ISSN 0028-3878. PMID 24174585. https://n.neurology.org/content/81/22/1962.
- ↑ McKeon, Andrew; Martinez-Hernandez, Eugenia; Lancaster, Eric; Matsumoto, Joseph Y.; Harvey, Robert J.; McEvoy, Kathleen M.; Pittock, Sean J.; Lennon, Vanda A. et al. (January 2013). "Glycine Receptor Autoimmune Spectrum With Stiff-Man Syndrome Phenotype". JAMA Neurology 70 (1): 44–50. doi:10.1001/jamaneurol.2013.574. PMID 23090334. PMC 3718477. https://jamanetwork.com/journals/jamaneurology/fullarticle/1384978. Retrieved 2022-12-09.
- ↑ Ariño, Helena; Gresa-Arribas, Nuria; Blanco, Yolanda; Martínez-Hernández, Eugenia; Sabater, Lidia; Petit-Pedrol, Mar; Rouco, Idoia; Bataller, Luis et al. (August 2014). "Cerebellar Ataxia and Glutamic Acid Decarboxylase Antibodies". JAMA Neurology 71 (8): 1009–1016. doi:10.1001/jamaneurol.2014.1011. PMID 24934144. PMC 4841264. https://jamanetwork.com/journals/jamaneurology/fullarticle/1881114. Retrieved 2022-12-09.
- ↑ Martinez-Hernandez, Eugenia; Sepulveda, Maria; Rostásy, Kevin; Höftberger, Romana; Graus, Francesc; Harvey, Robert J.; Saiz, Albert; Dalmau, Josep (February 2015). "Antibodies to Aquaporin 4, Myelin-Oligodendrocyte Glycoprotein, and the Glycine Receptor α1 Subunit in Patients With Isolated Optic Neuritis". JAMA Neurology 72 (2): 187–193. doi:10.1001/jamaneurol.2014.3602. PMID 25506781. PMC 4836943. https://jamanetwork.com/journals/jamaneurology/fullarticle/2022387. Retrieved 2022-12-09.
- ↑ Piepgras, Johannes; Höltje, Markus; Michel, Klaus; Li, Qin; Otto, Carolin; Drenckhahn, Christoph; Probst, Christian; Schemann, Michael et al. (8 September 2015). "Anti-DPPX encephalitis: Pathogenic effects of antibodies on gut and brain neurons" (in en). Neurology 85 (10): 890–897. doi:10.1212/WNL.0000000000001907. ISSN 0028-3878. PMID 26291285. PMC 4560062. https://n.neurology.org/content/85/10/890.
- ↑ 27.0 27.1 Boronat, Anna; Gelfand, Jeffrey M.; Gresa-Arribas, Nuria; Jeong, Hyo-Young; Walsh, Michael; Roberts, Kirk; Martinez-Hernandez, Eugenia; Rosenfeld, Myrna R. et al. (January 2013). "Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels" (in en). Annals of Neurology 73 (1): 120–128. doi:10.1002/ana.23756. PMID 23225603.
- ↑ Sabater, Lidia; Gaig, Carles; Gelpi, Ellen; Bataller, Luis; Lewerenz, Jan; Torres-Vega, Estefanía; Contreras, Angeles; Giometto, Bruno et al. (1 June 2014). "A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study" (in English). The Lancet Neurology 13 (6): 575–586. doi:10.1016/S1474-4422(14)70051-1. ISSN 1474-4422. PMID 24703753.
- ↑ Gaig, Carles; Graus, Francesc; Compta, Yarko; Högl, Birgit; Bataller, Luis; Brüggemann, Norbert; Giordana, Caroline; Heidbreder, Anna et al. (2 May 2017). "Clinical manifestations of the anti-IgLON5 disease" (in en). Neurology 88 (18): 1736–1743. doi:10.1212/WNL.0000000000003887. ISSN 0028-3878. PMID 28381508. PMC 5409845. https://n.neurology.org/content/88/18/1736.
- ↑ Lopez-Chiriboga, A. Sebastian; Komorowski, Lars; Kümpfel, Tania; Probst, Christian; Hinson, Shannon R.; Pittock, Sean J.; McKeon, Andrew (15 March 2016). "Metabotropic glutamate receptor type 1 autoimmunity: Clinical features and treatment outcomes" (in en). Neurology 86 (11): 1009–1013. doi:10.1212/WNL.0000000000002476. ISSN 0028-3878. PMID 26888994. PMC 4799712. https://n.neurology.org/content/86/11/1009.
- ↑ 31.0 31.1 Lancaster, E.; Martinez-Hernandez, E.; Titulaer, M. J.; Boulos, M.; Weaver, S.; Antoine, J.-C.; Liebers, E.; Kornblum, C. et al. (1 November 2011). "Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome" (in en). Neurology 77 (18): 1698–1701. doi:10.1212/WNL.0b013e3182364a44. ISSN 0028-3878. PMID 22013185. PMC 3208954. https://n.neurology.org/content/77/18/1698.
External links
| Classification | |
|---|---|
| External resources |
 |
