Medicine:Erdheim–Chester disease
Erdheim–Chester disease (ECD) is an extremely rare disease characterized by the abnormal multiplication of a specific type of white blood cells called histiocytes, or tissue macrophages (technically, this disease is termed a non-Langerhans-cell histiocytosis). It was declared a histiocytic neoplasm by the World Health Organization in 2016.[1] Onset typically is in middle age, although younger patients have been documented. The disease involves an infiltration of lipid-laden macrophages, multinucleated giant cells, an inflammatory infiltrate of lymphocytes and histiocytes in the bone marrow, and a generalized sclerosis of the long bones.[2]
Signs and symptoms
Long bone involvement is almost universal in ECD patients and is bilateral and symmetrical in nature. More than 50% of cases have some sort of extraskeletal involvement. This can include kidney, skin, brain and lung involvement, and less frequently retroorbital tissue, pituitary gland and heart involvement is observed.[3]
Bone pain is the most frequent of all symptoms associated with ECD and mainly affects the lower limbs, knees and ankles. The pain is often described as mild but permanent, and juxtaarticular in nature. Exophthalmos occurs in some patients and is usually bilateral, symmetric and painless, and in most cases it occurs several years before the final diagnosis. Recurrent pericardial effusion can be a manifestation,[4] as can morphological changes in adrenal size and infiltration.[5]
A review of 59 case studies by Veyssier-Belot et al. in 1996 reported the following symptoms in order of frequency of occurrence:[6]
- Bone pain
- Retroperitoneal fibrosis
- Diabetes insipidus
- Exophthalmos
- Xanthomas
- Neurological and central nervous system involvement
- Dyspnea caused by interlobular septal and pleural thickening
- Kidney failure
- Hypopituitarism
- Liver failure
Diagnosis
Radiologic osteosclerosis and histology are the main diagnostic features. Diagnosis can often be difficult because of the rareness of ECD as well as the need to differentiate it from LCH. A diagnosis from neurological imaging may not be definitive. The presence of symmetrical cerebellar and pontine signal changes on T2-weighted images seem to be typical of ECD, however, multiple sclerosis and metabolic diseases must also be considered in the differential diagnosis.[7] ECD is not a common cause of exophthalmos but can be diagnosed by biopsy. However, like all biopsies, this may be inconclusive.[8] Video-assisted thoracoscopic surgery may be used for diagnostic confirmation and also for therapeutic relief of recurrent pericardial fluid drainage.[9]
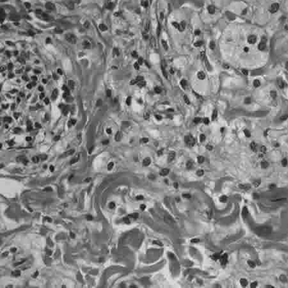
Histology
Histologically, ECD differs from Langerhans cell histiocytosis (LCH) in a number of ways. Unlike LCH, ECD does not stain positive for S-100 proteins or Group 1 CD1a glycoproteins, and electron microscopy of cell cytoplasm does not disclose Birbeck granules.[6] Tissue samples show xanthomatous or xanthogranulomatous infiltration by lipid-laden or foamy histiocytes, and are usually surrounded by fibrosis. Bone biopsy is said to offer the greatest likelihood of reaching a diagnosis. It would appear that approximately half these patients harbor point mutations of the BRAF gene at codon 600 substituting the amino acid glutamine for valine. In some, there is histiocyte proliferation, and on staining, the section is CD68+ and CD1a-.[citation needed]
Treatment
There are two FDA-approved targeted drugs to treat ECD.
- Vemurafenib, an oral agent approved in 2019, targets the BRAF protein. It was approved after showing dramatic efficacy in ECD patients harboring the BRAF V600E mutation.[10][11]
- Cobimetinib, an oral inhibitor of MEK1 and MEK2, was approved in November 2022.[12]
Other treatment options include:
- Interferon-α[8]
- High-dose corticosteroid therapy
- Chemotherapy
- Pexidartinib, a drug that targets a mutation in the CSF1R pathway and has shown sustained, complete response in limited use.[13]
- Radiation therapy
- Surgical debulking
- Ciclosporin
Prognosis
Erdheim–Chester disease was previously associated with high mortality rates.[14] However, long-term survival is now more promising. Recent studies have reported that some patients receiving targeted therapies showed no disease progression. Targeted therapies using BRAF, MEK and/or other inhibitors have been dramatically efficacious.[10][13][15][16] In 2019, the Mayo Clinic published guidelines for the diagnosis and treatment of the disease, stressing the importance of genetic testing: "Recent insights into their genomic architecture demonstrating mitogen-activated protein kinase/extracellular signal-regulated kinase pathway mutations have now enabled potential treatment with targeted therapies in most patients."[17]
Epidemiology
Approximately 500 cases had been reported in the literature as of 2014.[18] ECD affects predominantly adults, with a mean age of 53 years.[6]
History
The first case of ECD was reported by the American pathologist William Chester in 1930, during his visit to the Austrian pathologist Jakob Erdheim in Vienna.[19]
Society and culture
The Erdheim–Chester Disease Global Alliance is a support and advocacy group with the goal of raising awareness of and promoting research into ECD.[20][21] ECD families and patients are also supported by the Histiocytosis Association, Inc.[21][22]
Media
In the TV show House, season 2 episode 17, "All In", the final diagnosis of a 6-year-old boy who presents with bloody diarrhea and ataxia is Erdheim–Chester disease.[23]
References
- ↑ "Erdheim-Chester Disease Declared a Histiocytic Neoplasm". 18 May 2016. http://erdheim-chester.org/wp-content/uploads/2015/05/ECD-Declared-a-Histiocytic-Neoplasm_FINAL1.pdf.
- ↑ "Erdheim–Chester disease". Medical Subject Headings. United States National Library of Medicine. 8 July 2008. https://meshb.nlm.nih.gov/record/ui?ui=D031249.
- ↑ "Erdheim-Chester Disease" (in en-us). Histiocytosis Association. https://www.histio.org/page.aspx?pid=403.
- ↑ "[Recurrent pericardial effusion as first manifestation of Erdheim-Chester disease]" (in de). Deutsche Medizinische Wochenschrift 136 (39): 1952–1956. September 2011. doi:10.1055/s-0031-1286368. PMID 21935854.
- ↑ "Bilateral adrenal infiltration in Erdheim-Chester disease. Report of seven cases and literature review". The Journal of Clinical Endocrinology and Metabolism 92 (6): 2007–2012. June 2007. doi:10.1210/jc.2006-2018. PMID 17405844.
- ↑ Jump up to: 6.0 6.1 6.2 "Erdheim-Chester disease. Clinical and radiologic characteristics of 59 cases". Medicine 75 (3): 157–169. May 1996. doi:10.1097/00005792-199605000-00005. PMID 8965684.
- ↑ "Cerebral Erdheim-Chester disease: case report and review of the literature". Neuroradiology 45 (4): 241–245. April 2003. doi:10.1007/s00234-003-0950-z. PMID 12687308.
- ↑ Jump up to: 8.0 8.1 "Erdheim Chester Disease". M. D. Anderson Cancer Center. http://www.mdanderson.org/patient-and-cancer-information/cancer-information/cancer-types/eye-cancer/index.html.
- ↑ "Erdheim-Chester disease: The role of video-assisted thoracoscopic surgery in diagnosing and treating cardiac involvement". International Journal of Surgery Case Reports 3 (3): 107–110. 2012. doi:10.1016/j.ijscr.2011.12.001. PMID 22288060.
- ↑ Jump up to: 10.0 10.1 "Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation". Blood 121 (9): 1495–1500. February 2013. doi:10.1182/blood-2012-07-446286. PMID 23258922.
- ↑ "FDA Approves First Treatment for Erdheim-Chester Disease". Pharmacy Practice News. 6 November 2017. http://www.pharmacypracticenews.com/FDA-Approvals/Article/11-17/FDA-Approves-First-Treatment-for-Erdheim-Chester-Disease/45182/.
- ↑ "FDA Approves Cobimetinib for Histiocytic Neoplasms" (in en). 2 November 2022. https://www.onclive.com/view/fda-approves-cobimetinib-for-histiocytic-neoplasms.
- ↑ Jump up to: 13.0 13.1 "Sustained, complete response to pexidartinib in a patient with CSF1R-mutated Erdheim-Chester disease". American Journal of Hematology 97 (3): 293–302. March 2022. doi:10.1002/ajh.26441. PMID 34978715.
- ↑ "Treatment of Erdheim-Chester disease with cladribine: a rational approach". The British Journal of Ophthalmology 88 (6): 844–847. June 2004. doi:10.1136/bjo.2003.035584. PMID 15148234.
- ↑ "Vemurafenib in the Treatment of Erdheim Chester Disease: A Systematic Review". Cureus 14 (6): e25935. June 2022. doi:10.7759/cureus.25935. PMID 35844342.
- ↑ The ASCO Post Staff (2 November 2022). "FDA Approves Oral MEK Inhibitor Cobimetinib for Histiocytic Neoplasms". The ASCO Post. https://ascopost.com/news/november-2022/fda-approves-oral-mek-inhibitor-cobimetinib-for-histiocytic-neoplasms/.
- ↑ "The Mayo Clinic Histiocytosis Working Group Consensus Statement for the Diagnosis and Evaluation of Adult Patients With Histiocytic Neoplasms: Erdheim-Chester Disease, Langerhans Cell Histiocytosis, and Rosai-Dorfman Disease". Mayo Clinic Proceedings 94 (10): 2054–2071. October 2019. doi:10.1016/j.mayocp.2019.02.023. PMID 31472931.
- ↑ "Erdheim-Chester disease". Current Rheumatology Reports 16 (4): 412. April 2014. doi:10.1007/s11926-014-0412-0. PMID 24532298.
- ↑ "Über Lipoidgranulomatose". Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin 279 (2): 561–602. 1930. doi:10.1007/BF01942684.
- ↑ "Erdheim–Chester Disease". ECD Global Alliance. http://www.erdheim-chester.org.
- ↑ Jump up to: 21.0 21.1 "Erdheim Chester disease" (in en-US). http://rarediseases.org/rare-diseases/erdheim-chester-disease/#supporting-organizations.
- ↑ "What Do I Do Now? - Erdheim-Chester Disease". Histiocytosis Association. http://www.histio.org/page.aspx?pid=592#Creatingasupportnetwork.
- ↑ "Internet Movie Database". https://www.imdb.com/title/tt0774235/?ref_=ttep_ep17.
Further reading
- "Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease". Blood 116 (20): 4070–4076. November 2010. doi:10.1182/blood-2010-04-279240. PMID 20724540.
- "18F-fluorodeoxyglucose-positron emission tomography scanning is more useful in followup than in the initial assessment of patients with Erdheim-Chester disease". Arthritis and Rheumatism 60 (10): 3128–3138. October 2009. doi:10.1002/art.24848. PMID 19790052.
- "Pulmonary involvement in Erdheim-Chester disease: a single-center study of thirty-four patients and a review of the literature". Arthritis and Rheumatism 62 (11): 3504–3512. November 2010. doi:10.1002/art.27672. PMID 20662053.
- "Treatment of refractory Erdheim-Chester disease with double autologous hematopoietic stem-cell transplantation". Annals of Internal Medicine 135 (9): 844–845. November 2001. doi:10.7326/0003-4819-135-9-200111060-00027. PMID 11694122.
- "Successful treatment of Erdheim-Chester disease, a non-Langerhans-cell histiocytosis, with interferon-alpha". Blood 106 (9): 2992–2994. November 2005. doi:10.1182/blood-2005-06-2238. PMID 16020507.
- "Erdheim-Chester disease: CT findings of thoracic involvement". European Radiology 20 (11): 2579–2587. November 2010. doi:10.1007/s00330-010-1830-7. PMID 20563815.
- "Erdheim-Chester disease: case report with unique postmortem magnetic resonance imaging, high-resolution radiography, and pathologic correlation". Clinical Imaging 33 (2): 150–153. 2009. doi:10.1016/j.clinimag.2008.09.009. PMID 19237062.
- "Cerebral, facial, and orbital involvement in Erdheim-Chester disease: CT and MR imaging findings". Radiology 255 (2): 586–594. May 2010. doi:10.1148/radiol.10090320. PMID 20413768.
- "Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature review". Medicine 83 (6): 371–392. November 2004. doi:10.1097/01.md.0000145368.17934.91. PMID 15525849.
- "Images in cardiovascular medicine. Cardiac involvement in Erdheim-Chester disease: magnetic resonance and computed tomographic scan imaging in a monocentric series of 37 patients". Circulation 119 (25): e597–e598. June 2009. doi:10.1161/CIRCULATIONAHA.108.825075. PMID 19564564.
- "Variability in the efficacy of interferon-alpha in Erdheim-Chester disease by patient and site of involvement: results in eight patients". Arthritis and Rheumatism 54 (10): 3330–3336. October 2006. doi:10.1002/art.22165. PMID 17009306.
- "Imatinib mesylate for platelet-derived growth factor receptor-beta-positive Erdheim-Chester histiocytosis". Blood 111 (11): 5413–5415. June 2008. doi:10.1182/blood-2008-03-148304. PMID 18502845.
- "Response of histiocytoses to imatinib mesylate: fire to ashes". Journal of Clinical Oncology 28 (31): e633–e636. November 2010. doi:10.1200/JCO.2010.29.9073. PMID 20733125.
- "Neurological manifestations and neuroradiological presentation of Erdheim-Chester disease: report of 6 cases and systematic review of the literature". Journal of Neurology 253 (10): 1267–1277. October 2006. doi:10.1007/s00415-006-0160-9. PMID 17063320.
- "Biochemical markers of bone turnover, serum levels of interleukin-6/interleukin-6 soluble receptor and bisphosphonate treatment in Erdheim-Chester disease". Clinical and Experimental Rheumatology 21 (2): 232–236. 2003. PMID 12747282.
- "Erdheim-Chester disease with predominant mesenteric localization: lack of efficacy of interferon alpha". Joint Bone Spine 76 (3): 315–317. May 2009. doi:10.1016/j.jbspin.2008.09.013. PMID 19119043.
External links
 |