Medicine:Familial Mediterranean fever
| Familial Mediterranean fever | |
|---|---|
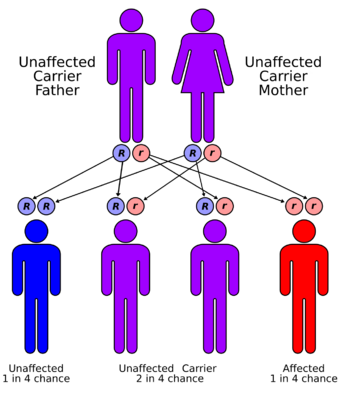 | |
| Familial Mediterranean fever has an autosomal recessive pattern of inheritance | |
| Specialty | Rheumatology, Immunology |
Familial Mediterranean fever (FMF) is a hereditary inflammatory disorder.[1]:149 FMF is an autoinflammatory disease caused by mutations in Mediterranean fever gene, which encodes a 781–amino acid protein called pyrin.[2] While all ethnic groups are susceptible to FMF, it usually occurs in people of Mediterranean origin—including Sephardic Jews, Mizrahi Jews, Ashkenazi Jews,[3][4] Assyrians, Armenians, Azerbaijanis, Druze, Levantines, Kurds, Greeks, Turks and Italians.[5][6][7][8]
The disorder has been given various names, including familial paroxysmal polyserositis, periodic peritonitis, recurrent polyserositis, benign paroxysmal peritonitis, periodic disease or periodic fever, Reimann periodic disease or Reimann syndrome, Siegal-Cattan-Mamou disease, and Wolff periodic disease.[9][10][11] Note that "periodic fever" can also refer to any of the periodic fever syndromes.
Signs and symptoms
Attacks
There are seven types of attacks. Ninety percent of all patients have their first attack before they are 18 years old. All develop over 2–4 hours and last anywhere from 6 hours to 5 days. Most attacks involve fever.[12]
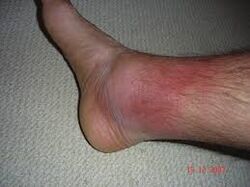
- Abdominal attacks, featuring abdominal pain, affect the whole abdomen with all signs of peritonitis (inflammation of abdominal lining), and acute abdominal pain like appendicitis. They occur in 95% of all patients and may lead to unnecessary laparotomy. Incomplete attacks, with local tenderness and normal blood tests, have been reported.
- Joint attacks mainly occur in large joints, especially in the legs. Usually, only one joint is affected. 75% of all FMF patients experience joint attacks.
- Chest attacks include pleuritis (inflammation of the pleura) and pericarditis (inflammation of the pericardium). Pleuritis occurs in 40% of patients and makes it difficult to breathe or lie flat, but pericarditis is rare.
- Scrotal attacks due to inflammation of the tunica vaginalis are somewhat rare but may be mistaken for testicular torsion.
- Myalgia (rare in isolation)
- Erysipeloid rashes (a skin reaction on the legs that can mimic cellulitis, rare in isolation)
Diagnostic criteria
Various diagnostic criteria have been set, but the Tel-Hashomer clinical criteria is widely recognized for the diagnosis of FMF. It has more than 95% and 97% sensitivity and specificity, respectively.[13]
For the criteria, typical attacks consist of all the following: recurrent (three or more episodes), febrile (rectal ≥ 38 °C), painful inflammation, and a short duration of 12 to 72 hours.
Incomplete attacks (must be recurrent) are differing from typical attacks in at least one feature as follows: temperature <38 °C, attack duration longer or shorter than specified (but no less than 6 hours and no more than 7 days), localized abdominal attacks, no signs of peritonitis during the attacks, and arthritis in a location other than the hip, knee or ankle.
Complications
AA-amyloidosis with kidney failure is a complication and may develop without overt crises. AA amyloid protein is produced in very large quantities during attacks, and at a low rate between them, and accumulates mainly in the kidney, as well as the heart, spleen, gastrointestinal tract, and thyroid.[12]
There appears to be an increase in the risk for developing particular vasculitis-related diseases[14] (e.g. Henoch–Schönlein purpura, Polyarteritis nodosa, and Behçet's disease), spondylarthropathy, prolonged arthritis of certain joints and protracted myalgia.[12]
Genetics
The MEFV gene is located on the short arm of chromosome 16 (16p13). Many different mutations of the MEFV gene can cause the disorder. Having one mutation is unlikely to cause the condition. Having two mutations (either a copy from both parents, or two different mutations with one from each parent) is the threshold for a genetic diagnosis of FMF. However, most individuals who comply with the genetic diagnosis of FMF remain asymptomatic or undiagnosed. Whether this is due to modifier genes or environmental factors remains to be established.[4]
Pathophysiology
Virtually all cases are due to a mutation in the Mediterranean Fever (MEFV) gene on the chromosome 16, which codes for a protein called pyrin or marenostrin. Various mutations of this gene lead to FMF, although some mutations cause a more severe picture than others. Mutations occur mainly in exons 2, 3, 5 and 10.[12]
The function of pyrin has not been completely elucidated, but in short, it is a protein that binds to the adaptor ASC and the pro form of the enzyme caspase-1 to generate multiprotein complexes called inflammasomes in response to certain infections. In healthy individuals, pyrin-mediated inflammasome assembly (which leads to the caspase 1) dependent processing and secretion of the pro-inflammatory cytokines (such as interleukin-18 (IL-18) and IL-1β) is a response to enterotoxins from certain bacteria.[15] The gain-of-function mutations in the MEFV gene render Pyrin hyperactive, and subsequently, the formation of the inflammasomes becomes more frequent.[16]
The pathophysiology of familial Mediterranean fever has recently undergone significant advances: at basal state, pyrin is kept inactive by a chaperone protein (belonging to the family of 14.3.3 proteins) linked to pyrin through phosphorylated serine residues.[17][18] The dephosphoration of pyrin is an essential prerequisite for the activation of the pyrin inflammasome. Inactivation of RhoA GTPases (by bacterial toxins, for example) leads to the inactivation of PKN1 / PKN2 kinases and dephosphoration of pyrin.[19] In healthy subjects, the dephosphorylation step alone does not cause activation of the pyrin inflammasome. In contrast, in FMF patients, the dephosphorylation of serines is sufficient to trigger the activation of the pyrin inflammasome.[20] This suggests that there is a two-level regulation and that the second regulatory mechanism (independent of (de)phosphorylation) is deficient in FMF patients. This deficient mechanism is probably located at the level of the B30.2 domain (exon 10) where most of the pathogenic mutations associated with FMF are located. It is probably the interaction of this domain with the cytoskeleton (microtubules) that is failing, as suggested by the efficacy of colchicine.[21]
It is not conclusively known what exactly sets off the attacks, and why overproduction of IL-1 would lead to particular symptoms in particular organs (e.g. joints or the peritoneal cavity).[12] However, steroid hormone catabolites (pregnanolone and etiocholanolone) have been shown to activate the pyrin inflammasome, in vitro, by interacting with the B30.2 domain (coded by exon 10).[22]
Diagnosis
The diagnosis is clinically made on the basis of the history of typical attacks, especially in patients from the ethnic groups in which FMF is more highly prevalent. An acute phase response is present during attacks, with high C-reactive protein levels, an elevated white blood cell count and other markers of inflammation. In patients with a long history of attacks, monitoring the kidney function is of importance in predicting chronic kidney failure.[12]
A genetic test is also available to detect mutations in the MEFV gene. Sequencing of exons 2, 3, 5, and 10 of this gene detects an estimated 97% of all known mutations.[12]
A specific and highly sensitive test for FMF is the "metaraminol provocative test (MPT)", whereby a single 10 mg infusion of metaraminol is administered to the patient. A positive diagnosis is made if the patient presents with a typical, albeit milder, FMF attack within 48 hours. As MPT is more specific than sensitive, it does not identify all cases of FMF, although a positive MPT can be very useful.[23][24]
Treatment
Attacks are self-limiting, and require analgesia and NSAIDs (such as diclofenac).[12] Colchicine, a drug otherwise mainly used in gout, decreases attack frequency in FMF patients. The exact way in which colchicine suppresses attacks is unclear. While this agent is not without side effects (such as abdominal pain and muscle pains), it may markedly improve quality of life in patients. The dosage is typically 1–2 mg a day. Development of amyloidosis is delayed with colchicine treatment. Interferon is being studied as a therapeutic modality.[12] Some advise discontinuation of colchicine before and during pregnancy, but the data are inconsistent, and others feel it is safe to take colchicine during pregnancy.[25]
Approximately 5–10% of FMF cases are resistant to colchicine therapy alone. In these cases, adding anakinra to the daily colchicine regimen has been successful.[26] Canakinumab, an anti-interleukin-1-beta monoclonal antibody, has likewise been shown to be effective in controlling and preventing flare-ups in patients with colchicine-resistant FMF and in two additional autoinflammatory recurrent fever syndromes: mevolonate kinase deficiency (hyper-immunoglobulin D syndrome, or HIDS) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS).[27]
Epidemiology
FMF affects groups of people originating from around the Levant or Eastern Mediterranean (hence its name); it is thus most prominent among those from or with ancestry from the regions including Arabs, Armenians, Jews (particularly Sephardi, Mizrahi, and to a lesser degree Ashkenazi Jews), and Turks.[3][12][28][29]
History
A New York City allergist, Sheppard Siegal, first described the attacks of peritonitis in 1945; he termed this "benign paroxysmal peritonitis", as the disease course was essentially benign.[30] Dr Hobart Reimann, working in the American University in Beirut, described a more complete picture which he termed "periodic disease".[31][32] French physicians Henry Mamou and Roger Cattan described the complete disease with renal complications in 1952.[33][34]
See also
- List of cutaneous conditions
- Urticarial syndromes
References
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology (10th ed.). Saunders. ISBN 0-7216-2921-0.
- ↑ "The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-kappaB through its N-terminal fragment". Blood 112 (5): 1794–803. September 2008. doi:10.1182/blood-2008-01-134932. PMID 18577712.
- ↑ 3.0 3.1 "Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups". European Journal of Human Genetics 8 (4): 307–10. April 2000. doi:10.1038/sj.ejhg.5200446. PMID 10854115.
- ↑ 4.0 4.1 "Familial Mediterranean fever: prevalence, penetrance and genetic drift". European Journal of Human Genetics 9 (8): 634–7. August 2001. doi:10.1038/sj.ejhg.5200672. PMID 11528510.
- ↑ "Familial Mediterranean fever". http://www.mayoclinic.org/diseases-conditions/familial-mediterranean-fever/basics/definition/con-20025734.
- ↑ "MEFV heterogeneity in Turkish Familial Mediterranean Fever patients". Molecular Biology Reports 37 (1): 355–8. January 2010. doi:10.1007/s11033-009-9779-9. PMID 19714479.
- ↑ Saeed, Dastgiri; Mortaza, Bonyadi; Tooba, Mizani (15 December 2010). "The Prevalence of Genetic Disorders in East Azerbaijan Province". Urmia Medical Journal 21 (4): 339–346. http://umj.umsu.ac.ir/browse.php?a_id=787&sid=1&slc_lang=en.
- ↑ "Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups". European Journal of Human Genetics 8 (4): 307–10. April 2000. doi:10.1038/sj.ejhg.5200446. PMID 10854115. http://troca.tbzmed.ac.ir/uploads/82/CMS/user/file/145/genetic/Higher%20than%20expected%20carrier%20rates%20for%20familial.pdf.
- ↑ Dugdale III, David C; Vyas, Jatin (2010-09-15). "Familial Mediterranean fever - PubMed Health". PubMed Health. National Centre for Biotechnology Information. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001402/.
- ↑ "Siegal-Cattan-Mamou syndrome". http://www.whonamedit.com/synd.cfm/2503.html.
- ↑ "Familial Mediterranean fever - Genetics Home Reference". Genetics Home Reference. U.S. National Library of Medicine. 2011-04-14. http://ghr.nlm.nih.gov/condition/familial-mediterranean-fever.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 12.9 "Diagnostic and treatment concerns in familial Mediterranean fever". Baillière's Best Practice & Research. Clinical Rheumatology 14 (3): 477–98. September 2000. doi:10.1053/berh.2000.0089. PMID 10985982.
- ↑ Livneh, Avi; Langevitz, Pnina; Zemer, Deborah; Zaks, Nurit; Kees, Salim; Lidar, Tzvi; Migdal, Amiel; Padeh, Shai et al. (1997). "Criteria for the diagnosis of familial mediterranean fever". Arthritis & Rheumatism 40 (10): 1879–1885. doi:10.1002/art.1780401023. PMID 9336425.
- ↑ Yalçınkaya, Fatoş; Özçakar, Z. Bı̇rsı̇n; Kasapçopur, Özgür; Öztürk, Ayşenur; Akar, Nejat; Bakkaloğlu, Ayşı̇n; Arısoy, Nı̇l; Ekı̇m, Mesı̇ha et al. (December 2007). "Prevalence of the MEFV Gene Mutations in Childhood Polyarteritis Nodosa". The Journal of Pediatrics 151 (6): 675–678. doi:10.1016/j.jpeds.2007.04.062. PMID 18035151.
- ↑ Alexandra A. Mushegian (2016). "An alternative path for pyrin". Science Signaling 9 (459): ec299. doi:10.1126/scisignal.aam6054. https://www.researchgate.net/publication/311780891.
- ↑ Ratner D (May 11, 2016). Activation and Inhibition of Multiple Inflammasome Pathways by the Yersinia Pestis Type Three Secretion System (Ph.D. thesis). University of Massachusetts Medical School.
- ↑ "Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice". Immunity 34 (5): 755–68. May 2011. doi:10.1016/j.immuni.2011.02.020. PMID 21600797.
- ↑ "The pyrin inflammasome: from sensing RhoA GTPases-inhibiting toxins to triggering autoinflammatory syndromes". Pathogens and Disease 76 (3). April 2018. doi:10.1093/femspd/fty020. PMID 29718184.
- ↑ "Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS". Nature Immunology 17 (8): 914–21. August 2016. doi:10.1038/ni.3457. PMID 27270401.
- ↑ "Pyrin dephosphorylation is sufficient to trigger inflammasome activation in familial Mediterranean fever patients". EMBO Molecular Medicine 11 (11): e10547. November 2019. doi:10.15252/emmm.201910547. PMID 31589380.
- ↑ "Familial Mediterranean fever mutations lift the obligatory requirement for microtubules in Pyrin inflammasome activation". Proceedings of the National Academy of Sciences of the United States of America 113 (50): 14384–14389. December 2016. doi:10.1073/pnas.1613156113. PMID 27911804. Bibcode: 2016PNAS..11314384V.
- ↑ Magnotti, Flora; Chirita, Daria; Dalmon, Sarah; Martin, Amandine; Bronnec, Pauline; Sousa, Jeremy; Helynck, Olivier; Lee, Wonyong et al. (2022). "Steroid hormone catabolites activate the pyrin inflammasome through a non-canonical mechanism". Cell Reports 41 (2): 111472. doi:10.1016/j.celrep.2022.111472. PMID 36223753.
- ↑ "Metaraminol provocative test: a specific diagnostic test for familial Mediterranean fever". Lancet 1 (8378): 656–7. March 1984. doi:10.1016/s0140-6736(84)92172-x. PMID 6142351.
- ↑ "[The metaraminol provocation test in the diagnosis of familial Mediterranean fever]". Monatsschrift Kinderheilkunde 136 (5): 243–5. May 1988. PMID 3405225.
- ↑ "Safety of colchicine therapy during pregnancy". Canadian Family Physician 49: 967–9. August 2003. PMID 12943352. PMC 2214270. http://www.cfpc.ca/cfp/2003/Aug/vol49-aug-clinical-1.asp.
- ↑ "The efficacy of anakinra in an adolescent with colchicine-resistant familial Mediterranean fever". European Journal of Pediatrics 167 (6): 695–6. June 2008. doi:10.1007/s00431-007-0547-3. PMID 17588171.
- ↑ "Canakinumab for the Treatment of Autoinflammatory Recurrent Fever Syndromes". The New England Journal of Medicine 378 (20): 1908–1919. May 2018. doi:10.1056/NEJMoa1706314. PMID 29768139. http://discovery.ucl.ac.uk/10049134/15/Brogan_nejmoa1706314.pdf.
- ↑ "Familial Mediterranean Fever". Acta Medica 57 (3): 97–104. 2014. doi:10.14712/18059694.2014.47. PMID 25649364.
- ↑ Sinha, Chandrasen K.; Davenport, Mark (2010). Handbook of Pediatric Surgery. New York: Springer. p. 192. ISBN 9781848821323.
- ↑ "Benign paroxysmal peritonitis". Annals of Internal Medicine 12 (2): 234–47. February 1949. doi:10.7326/0003-4819-23-1-1. PMID 18124924.
- ↑ "Periodic disease; a probable syndrome including periodic fever, benign paroxysmal peritonitis, cyclic neutropenia and intermittent arthralgia". Journal of the American Medical Association 136 (4): 239–44. January 1948. doi:10.1001/jama.1948.02890210023004. PMID 18920089.
- ↑ synd/2503 at Who Named It?
- ↑ Mamou, Henry; Cattan, Roger (1952). "Semaine Des Hôpitaux de Paris". La Maladie Périodique N°28: 1062–1070.
- ↑ "A brief history of familial Mediterranean fever". Saudi Medical Journal 36 (9): 1126–7. September 2015. doi:10.15537/smj.2015.9.12219. PMID 26318474.
External links
- Proteopedia 2wl1 information about the MEFV gene.
- GeneReview/NIH/UW entry on Familial Mediterranean Fever
- Familial Mediterranean Fever (FMF) - US National Institute of Arthritis and Musculoskeletal and Skin Diseases
| Classification | |
|---|---|
| External resources |
 |