Medicine:Septic shock
| Septic shock | |
|---|---|
 | |
| Sepsis is one of the most common causes of death in critically ill patients in intensive care units. Oil by Gabriël Metsu. | |
| Specialty | Infectious disease, critical care medicine, emergency medicine |
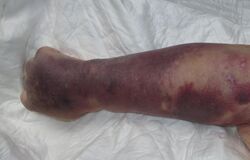
Septic shock is a potentially fatal medical condition that occurs when sepsis, which is organ injury or damage in response to infection, leads to dangerously low blood pressure and abnormalities in cellular metabolism. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defines septic shock as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by requiring a vasopressor to maintain a mean arterial pressure of 65 mm Hg or greater and having serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. This combination is associated with hospital mortality rates greater than 40%.[1]
The primary infection is most commonly caused by bacteria, but also may be caused by fungi, viruses, or parasites. It may be located in any part of the body, but most commonly in the lungs, brain, urinary tract, skin, or abdominal organs.[2] It can cause multiple organ dysfunction syndrome (formerly known as multiple organ failure) and death if not treated immediately.[3]
Frequently, people with septic shock are cared for in intensive care units. It most commonly affects children, immunocompromised individuals, and the elderly, as their immune systems cannot deal with infection as effectively as those of healthy adults. The mortality rate from septic shock is approximately 25–50%.[3]
Causes
Septic shock is a result of a systemic response to infection or multiple infectious causes. The precipitating infections that may lead to septic shock if severe enough include but are not limited to appendicitis, pneumonia, bacteremia, diverticulitis, pyelonephritis, meningitis, pancreatitis, necrotizing fasciitis, MRSA and mesenteric ischemia.[4][5]
According to the earlier definitions of sepsis updated in 2001,[6] sepsis is a constellation of symptoms secondary to an infection that manifests as disruptions in heart rate, respiratory rate, temperature, and white blood cell count. If sepsis worsens to the point of end-organ dysfunction (kidney failure, liver dysfunction, altered mental status, or heart damage), then the condition is called severe sepsis. In septic shock, events within tissue capillaries induce distributive shock in which the recovery of blood pressure is not achieved upon the administration of additional intravenous fluids, and requires a vasoconstrictive agent such as noradrenaline and/or vasopressin.[7]
Pathophysiology
The pathophysiology of septic shock is not entirely understood, but it is known that a key role in the development of severe sepsis is played by an immune and coagulation response to an infection. Both pro-inflammatory and anti-inflammatory responses play a role in septic shock.[8] Septic shock involves a widespread inflammatory response that produces a hypermetabolic effect. This is manifested by increased cellular respiration, protein catabolism, and metabolic acidosis with a compensatory respiratory alkalosis.[9]
Most cases of septic shock are caused by gram-positive bacteria,[10] followed by endotoxin-producing gram-negative bacteria, although fungal infections are an increasingly prevalent cause of septic shock.[9] Toxins produced by pathogens cause an immune response; in gram-negative bacteria these are endotoxins, which are bacterial membrane lipopolysaccharides (LPS).
Gram-positive
Gram-negative
In response to inflammation, a compensatory reaction of production of anti-inflammatory substances such as IL-4, IL-10 antagonists, IL-1 receptor, and cortisol occurs. This is called compensatory anti-inflammatory response syndrome (CARS).[11] Both the inflammatory and anti-inflammatory reactions are responsible for the course of sepsis and are described as MARS (Mixed Antagonist Response Syndrome). The aim of these processes is to keep inflammation at an appropriate level. CARS often leads to suppression of the immune system, which leaves patients vulnerable to secondary infection.[8] It was once thought that SIRS or CARS could predominate in a septic individual, and it was proposed that CARS follows SIRS in a two-wave process. It is now believed that the systemic inflammatory response and the compensatory anti-inflammatory response occur simultaneously.[11]
At high levels of LPS, the syndrome of septic shock supervenes; the same cytokine and secondary mediators, now at high levels, result in systemic vasodilation (hypotension), diminished myocardial contractility, widespread endothelial injury, activation causing systemic leukocyte adhesion and diffuse alveolar capillary damage in the lung, and activation of the coagulation system culminating in disseminated intravascular coagulation (DIC).
The hypoperfusion from the combined effects of widespread vasodilation, myocardial pump failure, and DIC causes multiorgan system failure that affects the liver, kidneys, and central nervous system, among other organ systems. Recently, severe damage to liver ultrastructure has been noticed from treatment with cell-free toxins of Salmonella.[12] Unless the underlying infection (and LPS overload) is rapidly brought under control, the patient usually dies. The ability of TLR4 to respond to a distinct LPS species is clinically important. Pathogenic bacteria may employ LPS with low biological activity to evade proper recognition by the TLR4/MD-2 system, dampening the host immune response and increasing the risk of bacterial dissemination. On the other hand, such LPS would not be able to induce septic shock in susceptible patients, rendering septic complications more manageable. Yet, defining and understanding how even the smallest structural differences between the very similar LPS species may affect the activation of the immune response may provide the mechanism for the fine tuning of the latter and new insights to immunomodulatory processes.[13]
Diagnosis
According to current guidelines, requirements for diagnosis with sepsis are "the presence (probable or documented) of infection together with systemic manifestations of infection".[9] These manifestations may include:
- Tachypnea (fast rate of breathing), which is defined as more than 20 breaths per minute, or when testing blood gas, a PaCO2 less than 32 mm Hg, which signifies hyperventilation
- White blood cell count either significantly low (< 4000 cells/mm3), or elevated (> 12000 cells/mm3)
- Tachycardia (rapid heart rate), which in sepsis is defined as a rate greater than 90 beats per minute
- Altered body temperature: Fever > 38.0 °C (100.4 °F) or hypothermia < 36.0 °C (96.8 °F)
Documented evidence of infection may include positive blood culture, signs of pneumonia on chest x-ray, or other radiologic or laboratory evidence of infection. Signs of end-organ dysfunction are present in septic shock, including kidney failure, liver dysfunction, changes in mental status, or elevated serum lactate.
Septic shock is diagnosed if there is low blood pressure (BP) that does not respond to treatment. This means that intravenous fluid administration alone is not enough to maintain a patient's BP. Diagnosis of septic shock is made when systolic blood pressure is less than 90 mm Hg, a mean arterial pressure (MAP) is less than 70 mm Hg, or a systolic BP decrease of 40 mm Hg or more without other causes for low BP.[9]
Definition
Septic shock is a subclass of distributive shock, a condition in which abnormal distribution of blood flow in the smallest blood vessels results in inadequate blood supply to the body tissues, resulting in ischemia and organ dysfunction. Septic shock refers specifically to distributive shock due to sepsis as a result of infection.[14]
Septic shock may be defined as sepsis-induced low blood pressure that persists despite treatment with intravenous fluids.[9] Low blood pressure reduces tissue perfusion pressure, causing the tissue hypoxia that is characteristic of shock. Cytokines released in a large-scale inflammatory response result in massive vasodilation, increased capillary permeability, decreased systemic vascular resistance, and low blood pressure. Finally, in an attempt to offset decreased blood pressure, ventricular dilatation and myocardial dysfunction occur.[citation needed]
Septic shock may be regarded as a stage of SIRS (Systemic Inflammatory Response Syndrome), in which sepsis, severe sepsis, and multiple organ dysfunction syndrome (MODS) represent different stages of a pathophysiological process. If an organism cannot cope with an infection, it may lead to a systemic response - sepsis, which may further progress to severe sepsis, septic shock, organ failure, and eventually, result in death.[citation needed]
Treatment
Treatment primarily consists of the following:
- Giving intravenous fluids[15]
- Early antibiotic administration [15]
- Early goal directed therapy[15]
- Rapid source identification and control
- Support of major organ dysfunction
Fluids
Because lowered blood pressure in septic shock contributes to poor perfusion, fluid resuscitation is an initial treatment to increase blood volume. Patients demonstrating sepsis-induced hypoperfusion should be initially resuscitated with at least 30 ml/kg of intravenous crystalloid within the first three hours.[5] Crystalloids such as normal saline and lactated Ringer's solution are recommended as the initial fluid of choice, while the use of colloid solutions such as hydroxyethyl starch have not shown any advantage or decrease in mortality. When large quantities of fluids are given, administering albumin has shown some benefit.[10] However, too high of a rate of fluid infusion can be more risky; the particular type of fluid's flow rate must be closely monitored, along with the patient's condition and vital signs.[16]
Antibiotics
Treatment guidelines call for the administration of broad-spectrum antibiotics within the first hour following recognition of septic shock. Prompt antimicrobial therapy is important, as the risk of dying increases by approximately 10% for every hour of delay in receiving antibiotics.[10] Time constraints do not allow the culture, identification, and testing for antibiotic sensitivity of the specific microorganism responsible for the infection. Therefore, combination antimicrobial therapy, which covers a wide range of potential causative organisms, is tied to better outcomes.[10] Antibiotics should be continued for 7–10 days in most patients, though treatment duration may be shorter or longer depending on clinical response.[11]
Vasopressors
Among the choices for vasopressors, norepinephrine is superior to dopamine in septic shock.[17] Norepinephrine is the preferred vasopressor, while epinephrine may be added to norepinephrine when needed. Low-dose vasopressin also may be used as an addition to norepinephrine, but is not recommended as a first-line treatment. Dopamine may cause rapid heart rate and arrhythmias, and is only recommended in combination with norepinephrine in those with slow heart rate and low risk of arrhythmia. In the initial treatment of low blood pressure in septic shock, the goal of vasopressor treatment is a mean arterial pressure (MAP) of 65 mm Hg.[10] In 2017, the FDA approved angiotensin II injection for intravenous infusion to increase blood pressure in adults with septic or other distributive shock.[18]
Methylene blue
Methylene blue is useful for this condition.[19][20][21][22] Although use of methylene blue has mostly been in adults it has also been shown to work in children.[23][24] Its mechanism of action is thought to be via the inhibition of the nitric oxide-cyclic guanosine monophosphate pathway.[25] This pathway is excessively activated in septic shock. Methylene blue has been found to work in cases resistant to the usual agents.[26] This effect was first reported in the early 1990s.[27][28]
Other
While there is tentative evidence for β-Blocker therapy to help control heart rate, evidence is not significant enough for its routine use.[29][30] There is tentative evidence that steroids may be useful in improving outcomes.[31]
Tentative evidence exists that Polymyxin B-immobilized fiber column hemoperfusion may be beneficial in the treatment of septic shock.[32] Trials are ongoing and it is currently being used in Japan and Western Europe.[33]
Recombinant activated protein C (drotrecogin alpha) in a 2011 Cochrane review was found not to decrease mortality and to increase bleeding, and thus, was not recommended for use.[34] Drotrecogin alfa (Xigris) was withdrawn from the market in October 2011.
Epidemiology
Sepsis has a worldwide incidence of more than 20 million cases a year, with mortality due to septic shock reaching up to 50 percent even in industrialized countries.[35]
35% of septic shock cases derive from urinary tract infections, 15% from the respiratory tract, 15% from skin catheters (such as IVs), and more than 30% of all cases are idiopathic in origin.[citation needed]
The mortality rate from sepsis, especially if it is not treated rapidly with the needed medications in a hospital, is approximately 40% in adults and 25% in children. It is significantly greater when sepsis is left untreated for more than seven days.[36]
References
- ↑ "The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)". JAMA 315 (8): 801–10. February 2016. doi:10.1001/jama.2016.0287. PMID 26903338.
- ↑ Jui, Jonathan (2011). "Ch. 146: Septic Shock". Tintinalli's Emergency Medicine: A Comprehensive Study Guide (7th ed.). New York: McGraw-Hill. pp. 1003–14. http://www.accessmedicine.com/content.aspx?aID=6364928. Retrieved December 11, 2012.
- ↑ 3.0 3.1 Kumar, V.; Abbas, A.K.; Fausto, N. et al., eds (2007). Robbins Basic Pathology (8th ed.). Saunders, Elsevier. pp. 102–3. ISBN 978-1-4160-2973-1.
- ↑ "Bowel necrosis associated with early jejunal tube feeding: A complication of postoperative enteral nutrition". Arch Surg 141 (7): 701–4. July 2006. doi:10.1001/archsurg.141.7.701. PMID 16847244.
- ↑ 5.0 5.1 "Enteral nutrition associated non-occlusive bowel ischemia". J Korean Surg Soc 83 (3): 171–4. September 2012. doi:10.4174/jkss.2012.83.3.171. PMID 22977764.
- ↑ for the International Sepsis Definitions Conference; Levy, Mitchell M.; Fink, Mitchell P.; Marshall, John C.; Abraham, Edward; Angus, Derek; Cook, Deborah; Cohen, Jonathan et al. (2003). "2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference" (in en). Intensive Care Medicine 29 (4): 530–538. doi:10.1007/s00134-003-1662-x. ISSN 0342-4642. PMID 12664219.
- ↑ Hotchkiss, Richard S.; Moldawer, Lyle L.; Opal, Steven M.; Reinhart, Konrad; Turnbull, Isaiah R.; Vincent, Jean-Louis (2016-06-30). "Sepsis and septic shock". Nature Reviews. Disease Primers 2: 16045. doi:10.1038/nrdp.2016.45. ISSN 2056-676X. PMID 28117397.
- ↑ 8.0 8.1 "Severe sepsis and septic shock". N. Engl. J. Med. 369 (9): 840–51. August 2013. doi:10.1056/NEJMra1208623. PMID 23984731.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012". Crit. Care Med. 41 (2): 580–637. February 2013. doi:10.1097/CCM.0b013e31827e83af. PMID 23353941.
- ↑ 10.0 10.1 10.2 10.3 10.4 "Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes". Expert Rev Anti Infect Ther 10 (6): 701–6. June 2012. doi:10.1586/eri.12.50. PMID 22734959.
- ↑ 11.0 11.1 11.2 "Compensatory anti-inflammatory response syndrome". Thromb. Haemost. 101 (1): 36–47. January 2009. doi:10.1160/TH08-07-0421. ISSN 0340-6245. PMID 19132187.
- ↑ YashRoy, R.C. (June 1994). "Liver damage by intra-ileal treatment with Salmonella 3,10:r:- extract as studied by light and electron microscopy". Indian Journal of Animal Sciences 64 (6): 597–99. https://www.researchgate.net/publication/230820149.(animal study).
- ↑ "Structural Relationship of the Lipid A Acyl Groups to Activation of Murine Toll-Like Receptor 4 by Lipopolysaccharides from Pathogenic Strains of Burkholderia mallei, Acinetobacter baumannii, and Pseudomonas aeruginosa". Front Immunol 6: 595. 2015. doi:10.3389/fimmu.2015.00595. PMID 26635809.
- ↑ "Septic Shock: Practice Essentials, Background, Pathophysiology". Medscape. 2024-06-26. https://emedicine.medscape.com/article/168402-overview?form=fpf.
- ↑ 15.0 15.1 15.2 Levinson, A.T.; Casserly, B.P.; Levy, M.M. (April 2011). "Reducing mortality in severe sepsis and septic shock". Seminars in Respiratory and Critical Care Medicine 32 (2): 195–205. doi:10.1055/s-0031-1275532. PMID 21506056.
- ↑ Meyhoff, Tine S. et al. (2022). "Restriction of Intravenous Fluid in ICU Patients with Septic Shock". New England Journal of Medicine 386 (26): 2459–2470. doi:10.1056/NEJMoa2202707. PMID 35709019.
- ↑ "Norepinephrine or dopamine for septic shock: systematic review of randomized clinical trials". J Intensive Care Med 27 (3): 172–8. 2012. doi:10.1177/0885066610396312. PMID 21436167.
- ↑ Commissioner, Office of the. "Press Announcements - FDA approves drug to treat dangerously low blood pressure" (in en). https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm590249.htm.
- ↑ Jang DH, Nelson LS, Hoffman RS (2013) Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol 9(3):242-249
- ↑ Baldo CF, Silva LM, Arcencio L, Albuquerque AAS, Celotto AC, Basile-Filho A, Evora PRB (2018) Why Methylene Blue have to be always present in the tocking of emergency antidotes. Curr Drug Targets 19(13):1550-1559
- ↑ McCartney SL, Duce L, Ghadimi K (2018) Intraoperative vasoplegia: methylene blue to the rescue! Curr Opin Anaesthesiol. 31(1):43-49
- ↑ Booth AT, Melmer PD, Tribble B, Mehaffey JH, Tribble C (2017) Methylene blue for vasoplegic syndrome. Heart Surg Forum 20(5):E234-E238
- ↑ Rutledge C, Brown B, Benner K, Prabhakaran P, Hayes L (2015) A novel use of methylene blue in the pediatric ICU. Pediatrics 136(4):e1030-4
- ↑ Volpon LC, Evora PRB, Teixeira GD, Godinho M, Scarpelini S, Carmona F, Carlotti APCP (2018) Methylene blue for refractory shock in polytraumatized patient: A case report. J Emerg Med 55(4):553-558
- ↑ Da Silva PS, Furtado P (2018) Methylene blue to treat refractory latex-induced anaphylactic shock: a case report. A A Pract 10(3):57-60
- ↑ Lo JC, Darracq MA, Clark RF (2014) A review of methylene blue treatment for cardiovascular collapse. J Emerg Med 46(5):670-679
- ↑ Schneider F, Lutun P, Hasselmann M, Stoclet JC, Tempé JD (1992) Methylene blue increases systemic vascular resistance in human septic shock. Preliminary observations. Intensive Care Med 18(5):309-11
- ↑ Preiser JC, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M, Kahn RJ, Vincent JL (1995) Methylene blue administration in septic shock: a clinical trial. Crit Care Med 23(2):259-264
- ↑ "Systematic review of use of β-blockers in sepsis". J Anaesthesiol Clin Pharmacol 31 (4): 460–5. 2015. doi:10.4103/0970-9185.169063. PMID 26702201.
- ↑ "Beta-blocker use in severe sepsis and septic shock: a systematic review". Curr Med Res Opin 31 (10): 1817–25. 2015. doi:10.1185/03007995.2015.1062357. PMID 26121122.
- ↑ Annane, Djillali; Bellissant, Eric; Bollaert, Pierre Edouard; Briegel, Josef; Keh, Didier; Kupfer, Yizhak; Pirracchio, Romain; Rochwerg, Bram (6 December 2019). "Corticosteroids for treating sepsis in children and adults". The Cochrane Database of Systematic Reviews 2019 (12). doi:10.1002/14651858.CD002243.pub4. ISSN 1469-493X. PMID 31808551.
- ↑ "Polymyxin B-immobilized fiber column hemoperfusion therapy for septic shock". Shock 36 (4): 332–8. October 2011. doi:10.1097/SHK.0b013e318225f839. PMID 21654557.
- ↑ "Polymyxin B hemoperfusion: a mechanistic perspective". Crit Care 18 (3): 309. June 2014. doi:10.1186/cc13912. PMID 25043934.
- ↑ "Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients". Cochrane Database Syst Rev 2018 (12). December 2012. doi:10.1002/14651858.CD004388.pub6. PMID 23235609.
- ↑ "Researchers make blood poisoning breakthrough". Phys.org. June 4, 2010. http://www.physorg.com/news194879092.html.
- ↑ Huether, S.E.; McCance, K.L., eds (2008). Understanding Pathophysiology (4th ed.). Mosby/Elsevier. ISBN 978-0-323-04990-0. https://archive.org/details/understandingpat04edhuet.
External links
| Classification | |
|---|---|
| External resources |
 |
