Biology:Interleukin 4
 Generic protein structure example |
| Interleukin 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
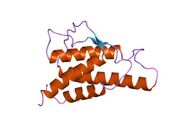
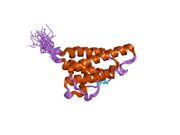
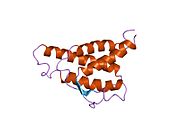
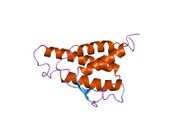
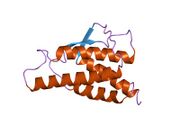
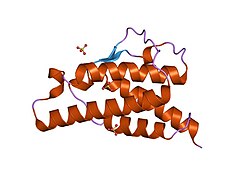
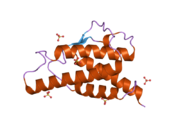
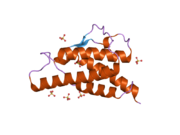
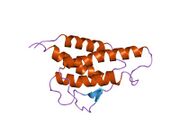
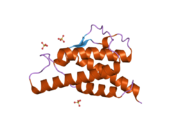
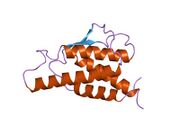
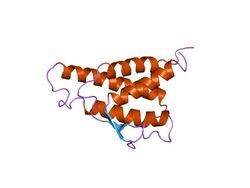 analysis of the solution structure of human interleukin 4 determined by heteronuclear three-dimensional nuclear magnetic resonance techniques | |||||||||
| Identifiers | |||||||||
| Symbol | IL4 | ||||||||
| Pfam | PF00727 | ||||||||
| Pfam clan | CL0053 | ||||||||
| InterPro | IPR002354 | ||||||||
| PROSITE | PDOC00655 | ||||||||
| SCOP2 | 2int / SCOPe / SUPFAM | ||||||||
| |||||||||
The interleukin 4 (IL4, IL-4) is a cytokine that induces differentiation of naive helper T cells (Th0 cells) to Th2 cells. Upon activation by IL-4, Th2 cells subsequently produce additional IL-4 in a positive feedback loop. IL-4 is produced primarily by mast cells, Th2 cells, eosinophils and basophils.[1] It is closely related and has functions similar to IL-13.
Function
Interleukin 4 has many biological roles, including the stimulation of activated B cell and T cell proliferation, and the differentiation of B cells into plasma cells. It is a key regulator in humoral and adaptive immunity. IL-4 induces B cell class switching to IgE, and up-regulates MHC class II production. IL-4 decreases the production of Th1 cells, macrophages, IFNγ, and dendritic cells IL-12.
Overproduction of IL-4 is associated with allergies.[2]
Inflammation and wound repair
Tissue macrophages play an important role in chronic inflammation and wound repair. The presence of IL-4 in extravascular tissues promotes alternative activation of macrophages into M2 cells and inhibits classical activation of macrophages into M1 cells. An increase in repair macrophages (M2) is coupled with secretion of IL-10 and TGF-β that result in a diminution of pathological inflammation. Release of arginase, proline, polyaminases and TGF-β by the activated M2 cell is tied with wound repair and fibrosis.[3]
Receptor
The receptor for interleukin-4 is known as the IL-4Rα. This receptor exists in 3 different complexes throughout the body. Type 1 receptors are composed of the IL-4Rα subunit with a common γ chain and specifically bind IL-4. Type 2 receptors consist of an IL-4Rα subunit bound to a different subunit known as IL-13Rα1. These type 2 receptors have the ability to bind both IL-4 and IL-13, two cytokines with closely related biological functions.[4][5]
Structure
IL-4 has a compact, globular fold (similar to other cytokines), stabilised by 3 disulphide bonds.[6] One half of the structure is dominated by a 4 alpha-helix bundle with a left-handed twist.[7] The helices are anti-parallel, with 2 overhand connections, which fall into a 2-stranded anti-parallel beta-sheet.[7]
Evolution
IL-4 is closely related to IL-13, and both stimulate type 2 immunity.[8] Genes of this family have also been found in fish, both in bony fish[9][10] and cartilaginous fish;[11] because at that evolutionary level they can't be distinguished as IL-4 or IL-13, they have been named IL-4/13.[10]
Discovery
This cytokine was co-discovered by Maureen Howard and William E. Paul[12] as well as by Ellen Vitetta and her research group in 1982.
The nucleotide sequence for human IL-4 was isolated four years later confirming its similarity to a mouse protein called B cell stimulatory factor-1 (BCSF-1).[13]
Animal studies
IL-4 has been found to mediate a crosstalk between the neural stem cells and neurons that undergo neurodegeneration, and initiate a regeneration cascade through phosphorylation of its intracellular effector STAT6 in an experimental Alzheimer's disease model in adult zebrafish brain.[14]
Clinical significance
IL-4 has also been shown to drive mitogenesis, dedifferentiation, and metastasis in rhabdomyosarcoma.[15] IL-4, along with other Th2 cytokines, is involved in the airway inflammation observed in the lungs of patients with allergic asthma.[16]
Illnesses associated with IL-4
IL-4 plays an important role in the development of certain immune disorders, particularly allergies and some autoimmune diseases.
Allergic diseases
Allergic diseases are sets of disorders that are manifested by a disproportionate response of the immune system to the allergen and Th2 responses. These pathologies include, for example, atopic dermatitis, asthma, or systemic anaphylaxis. Interleukin 4 mediates important pro-inflammatory functions in asthma, including induction of isotype rearrangement of IgE, expression of vascular cell adhesion molecule 1 (VCAM-1), promoting eosinophilic transmigration through endothelium, mucus secretion and T helper type 2 (Th2) leading to cytokine release. Asthma is a complex genetic disorder that has been associated with IL-4 gene promoter polymorphism and proteins involved in IL-4 signaling.[17]
Tumors
IL-4 has a significant effect on tumor progression. Increased IL-4 production was found in breast, prostate, lung, renal cells and other types of cancer. Overexpression of IL-4R has been found in many types of cancer. Renal cells and glioblastoma modify 10000–13000 receptors per cell depending on tumor type.[18]
IL-4 can primitively motivate tumor cells and increase their apoptosis resistance by increasing tumor growth.[19]
Nervous system
Brain tissue tumors such as astrocytoma, glioblastoma, meningioma, and medulloblastoma overexpress receptors for various growth factors including epidermal growth factor receptor, FGFR-1 (fibroblast growth factor receptor 1), TfR (transferrin receptor), IL-13R. Most human meningiomas massively expresses IL-4 receptors, indicating its role in cancer progression. They express IL-4Rα and IL13Rα-1-1, but not the surface γc chain, suggesting that most human meningiomas express IL-4 type II.[20]
HIV
IL-4 may also play a role in the infection and development of HIV disease. Auxiliary T cells are a key element of HIV-1 infection. Several signs of immune dysregulation such as polyclonal B cell initialization, previous cell-mediated antigen-induced response and hypergammaglobulinaemia occur in most HIV-1 infected patients and are associated with cytokines synthesized by Th2 cells. Increased IL-4 production by Th2 cells has been demonstrated in people infected with HIV.[21]
See also
References
- ↑ "IL-4 in the brain: a cytokine to remember". Journal of Immunology 189 (9): 4213–4219. November 2012. doi:10.4049/jimmunol.1202246. PMID 23087426.
- ↑ "The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor". The New England Journal of Medicine 337 (24): 1720–1725. December 1997. doi:10.1056/NEJM199712113372403. PMID 9392697.
- Lay summary in: "Scientists Identify Strong Genetic Link To Allergies". EurekAlert.org (Press release). December 10, 1997.
- ↑ Jon Aster; Vinay Kumar; Abul K. Abbas; Nelson Fausto (2009). Robbins & Cotran Pathologic Basis of Disease (8th ed.). Philadelphia: Saunders. p. 54. ISBN 978-1-4160-3121-5.
- ↑ "Targeting interleukin-4 in asthma: lost in translation?". American Journal of Respiratory Cell and Molecular Biology 47 (3): 261–270. September 2012. doi:10.1165/rcmb.2012-0080TR. PMID 22538865.
- ↑ "Interleukin-4 receptor signaling pathways in asthma pathogenesis". Trends in Molecular Medicine 10 (10): 493–499. October 2004. doi:10.1016/j.molmed.2004.08.004. PMID 15464449.
- ↑ "Disulfide assignments in recombinant mouse and human interleukin 4". Biochemistry 30 (6): 1515–1523. February 1991. doi:10.1021/bi00220a011. PMID 1993171.
- ↑ 7.0 7.1 "Crystal structure of recombinant human interleukin-4". The Journal of Biological Chemistry 267 (28): 20371–20376. October 1992. doi:10.2210/pdb2int/pdb. PMID 1400355.
- ↑ "T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production". Cytokine 75 (1): 14–24. September 2015. doi:10.1016/j.cyto.2015.05.010. PMID 26044597.
- ↑ "Cloning, characterization and expression analysis of pufferfish interleukin-4 cDNA: the first evidence of Th2-type cytokine in fish". Molecular Immunology 44 (8): 2078–2086. March 2007. doi:10.1016/j.molimm.2006.09.010. PMID 17084456.
- ↑ 10.0 10.1 "Comprehensive clarification of two paralogous interleukin 4/13 loci in teleost fish". Immunogenetics 60 (7): 383–397. July 2008. doi:10.1007/s00251-008-0299-x. PMID 18560827.
- ↑ "TH2 and Treg candidate genes in elephant shark". Nature 511 (7508): E7–E9. July 2014. doi:10.1038/nature13446. PMID 25008534.
- ↑ "Interleukins for B lymphocytes". Lymphokine Research 1 (1): 1–4. 1982. PMID 6985399.
- ↑ "Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities". Proceedings of the National Academy of Sciences of the United States of America 83 (16): 5894–5898. August 1986. doi:10.1073/pnas.83.16.5894. PMID 3016727. Bibcode: 1986PNAS...83.5894Y.
- ↑ "IL4/STAT6 Signaling Activates Neural Stem Cell Proliferation and Neurogenesis upon Amyloid-β42 Aggregation in Adult Zebrafish Brain". Cell Reports 17 (4): 941–948. October 2016. doi:10.1016/j.celrep.2016.09.075. PMID 27760324.
- ↑ "IL-4R drives dedifferentiation, mitogenesis, and metastasis in rhabdomyosarcoma". Clinical Cancer Research 17 (9): 2757–2766. May 2011. doi:10.1158/1078-0432.CCR-10-3445. PMID 21536546.
- ↑ "IL-4 and IL-13 signaling in allergic airway disease". Cytokine 75 (1): 68–78. September 2015. doi:10.1016/j.cyto.2015.05.014. PMID 26070934.
- ↑ "Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists". Respiratory Research 2 (2): 66–70. 19 February 2001. doi:10.1186/rr40. PMID 11686867.
- ↑ "Interleukin-4 receptor signaling and its binding mechanism: A therapeutic insight from inhibitors tool box". Cytokine & Growth Factor Reviews 32: 3–15. December 2016. doi:10.1016/j.cytogfr.2016.04.002. PMID 27165851.
- ↑ "Endogenous interleukin-4 promotes tumor development by increasing tumor cell resistance to apoptosis". Cancer Research 68 (21): 8687–8694. November 2008. doi:10.1158/0008-5472.CAN-08-0449. PMID 18974110.
- ↑ "Expression and structure of interleukin 4 receptors in primary meningeal tumors". Cancer 103 (10): 2132–2142. May 2005. doi:10.1002/cncr.21008. PMID 15830341.
- ↑ "Single cell analysis of IL-4 and IFN-gamma production by T cells from HIV-infected individuals: decreased IFN-gamma in the presence of preserved IL-4 production". Journal of Immunology 157 (6): 2712–2718. September 1996. doi:10.4049/jimmunol.157.6.2712. PMID 8805678.
Further reading
- "Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage". Progress in Neurobiology 178: 101610. July 2019. doi:10.1016/j.pneurobio.2019.03.003. PMID 30923023.
- "Interferon-gamma and interleukin-4 reciprocally regulate CD8 expression in CD8+ T cells". Proceedings of the National Academy of Sciences of the United States of America 105 (45): 17475–17480. November 2008. doi:10.1073/pnas.0809549105. PMID 18988742. Bibcode: 2008PNAS..10517475A.
- "Eosinophils and eosinophil-associated cytokines in allergic inflammation". International Archives of Allergy and Immunology 113 (1–3): 196–199. 1997. doi:10.1159/000237545. PMID 9130521.
- "Dysregulation of the IgE/Fc epsilon RI network in HIV-1 infection". The Journal of Allergy and Clinical Immunology 107 (1): 22–30. January 2001. doi:10.1067/mai.2001.111589. PMID 11149986.
- "Mechanisms of IgE elevation in HIV-1 infection". Critical Reviews in Immunology 20 (6): 477–496. 2001. doi:10.1615/critrevimmunol.v20.i6.40. PMID 11396683.
- "[Inflammatory cytokines (IL-4, IL-5 and IL-13)]". Nihon Rinsho. Japanese Journal of Clinical Medicine 59 (10): 1894–1899. October 2001. PMID 11676128.
- "IL-4 and IL-13: their pathological roles in allergic diseases and their potential in developing new therapies". Current Drug Targets. Inflammation and Allergy 1 (3): 263–269. September 2002. doi:10.2174/1568010023344661. PMID 14561191.
- "Modulation of HIV-1 transcription by cytokines and chemokines". Mini Reviews in Medicinal Chemistry 5 (12): 1093–1101. December 2005. doi:10.2174/138955705774933383. PMID 16375755.
- "The duplicitous effects of interleukin 4 on tumour immunity: how can the same cytokine improve or impair control of tumour growth?". Tissue Antigens 69 (4): 293–298. April 2007. doi:10.1111/j.1399-0039.2007.00831.x. PMID 17389011.
- "Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response". Nature Immunology 10 (7): 713–720. July 2009. doi:10.1038/ni.1738. PMID 19465907.
- "Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response". Nature Immunology 10 (7): 713–720. July 2009. doi:10.1038/ni.1738. PMID 19465907.
External links
- Interleukin-4 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Interleukin-4 from Gentaur
- Recombinant Human Interleukin-4 from Cornell University
- Interleukin-4 from Allergy Glossary at Health On the Net Foundation
- Overview of all the structural information available in the PDB for UniProt: P05112 (Interleukin-4) at the PDBe-KB.
 |