Physics:Extended X-ray absorption fine structure
X-ray Absorption Spectroscopy (XAS) includes both Extended X-Ray Absorption Fine Structure (EXAFS) and X-ray Absorption Near Edge Structure (XANES). XAS is the measurement of the x-ray absorption coefficient ([math]\displaystyle{ \mu (E) }[/math] in the equations below) of a material as a function of energy. X-rays of a narrow energy resolution are shone on the sample and the incident and transmitted x-ray intensity is recorded as the incident x-ray energy is incremented. The number of x-ray photons that are transmitted through a sample (It) is equal to the number of x-ray photons shone on the sample (I0) multiplied by a decreasing exponential that depends on the type of atoms in the sample, the absorption coefficient [math]\displaystyle{ \mu }[/math], and the thickness of the sample [math]\displaystyle{ x }[/math].
[math]\displaystyle{ I_t = I_0 e^{-\mu x} }[/math]
The absorption coefficient is obtained by taking the log ratio of the incident x-ray intensity to the transmitted x-ray intensity.
[math]\displaystyle{ \mu = \frac{-\mathrm{ln}({I}_{t}/{I}_{0})}{x} }[/math]
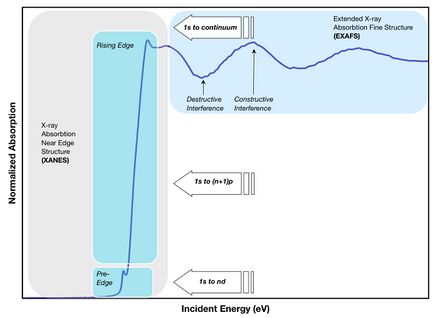
When the incident x-ray energy matches the binding energy of an electron of an atom within the sample, the number of x-rays absorbed by the sample increases dramatically, causing a drop in the transmitted x-ray intensity. This results in an absorption edge. Each element on the periodic table has a set of unique absorption edges corresponding to different binding energies of its electrons, giving XAS element selectivity. XAS spectra are most often collected at synchrotrons. Because X-rays are highly penetrating, XAS samples can be gases, solids or liquids. And because of the brilliance of synchrotron X-ray sources the concentration of the absorbing element can be as low as a few ppm.
EXAFS spectra are displayed as graphs of the absorption coefficient of a given material versus energy, typically in a 500 – 1000 eV range beginning before an absorption edge of an element in the sample. The x-ray absorption coefficient is usually normalized to unit step height. This is done by regressing a line to the region before and after the absorption edge, subtracting the pre-edge line from the entire data set and dividing by the absorption step height, which is determined by the difference between the pre-edge and post-edge lines at the value of E0 (on the absorption edge).
The normalized absorption spectra are often called XANES spectra. These spectra can be used to determine the average oxidation state of the element in the sample. The XANES spectra are also sensitive to the coordination environment of the absorbing atom in the sample. Finger printing methods have been used to match the XANES spectra of an unknown sample to those of known "standards". Linear combination fitting of several different standard spectra can give an estimate to the amount of each of the known standard spectra within an unknown sample.
X-ray absorption spectra are produced over the range of 200 – 35,000 eV. The dominant physical process is one where the absorbed photon ejects a core photoelectron from the absorbing atom, leaving behind a core hole. The atom with the core hole is now excited. The ejected photoelectron’s energy will be equal to that of the absorbed photon minus the binding energy of the initial core state. The ejected photoelectron interacts with electrons in the surrounding non-excited atoms.
If the ejected photoelectron is taken to have a wave-like nature and the surrounding atoms are described as point scatterers, it is possible to imagine the backscattered electron waves interfering with the forward-propagating waves. The resulting interference pattern shows up as a modulation of the measured absorption coefficient, thereby causing the oscillation in the EXAFS spectra. A simplified plane-wave single-scattering theory has been used for interpretation of EXAFS spectra for many years, although modern methods (like FEFF, GNXAS) have shown that curved-wave corrections and multiple-scattering effects can not be neglected. The photelectron scattering amplitude in the low energy range (5-200 eV) of the photoelectron kinetic energy become much larger so that multiple scattering events become dominant in the XANES (or NEXAFS) spectra.
The wavelength of the photoelectron is dependent on the energy and phase of the backscattered wave which exists at the central atom. The wavelength changes as a function of the energy of the incoming photon. The phase and amplitude of the backscattered wave are dependent on the type of atom doing the backscattering and the distance of the backscattering atom from the central atom. The dependence of the scattering on atomic species makes it possible to obtain information pertaining to the chemical coordination environment of the original absorbing (centrally excited) atom by analyzing these EXAFS data.
Experimental considerations
Since EXAFS requires a tunable x-ray source, data are always collected at synchrotrons, often at beamlines which are especially optimized for the purpose. The utility of a particular synchrotron to study a particular solid depends on the brightness of the x-ray flux at the absorption edges of the relevant elements.
Applications
XAS is an interdisciplinary technique and its unique properties, as compared to x-ray diffraction, have been exploited for understanding the details of local structure in:
- glass, amorphous and liquid systems
- solid solutions
- Doping and ionic implantation of materials for electronics
- local distortions of crystal lattices
- organometallic compounds
- metalloproteins
- metal clusters
- vibrational dynamics[citation needed]
- ions in solutions
- speciation of elements
Example of Significance
EXAFS is, like XANES, a highly sensitive technique with elemental specificity. As such, EXAFS is an extremely useful way to determine the chemical state of practically important species which occur in very low abundance or concentration. Frequent use of EXAFS occurs in environmental chemistry, where scientists try to understand the propagation of pollutants through an ecosystem. EXAFS can be used along with accelerator mass spectrometry in forensic examinations, particularly in nuclear non-proliferation applications.
For an example of an EXAFS study of uranium chemistry in glass see [1], and for a general study of trivalent lanthanides and actinides in chloride containing aqueous media can be read at [2]
See also
- X-ray absorption spectroscopy
- XANES/NEXAFS
- SEXAFS
History
A very detailed, balanced and informative account about the history of EXAFS (originally called Kossel's structures) is given in the paper "A History of the X-ray Absorption Fine Structure" by R. Stumm von Bordwehr, Ann. Phys. Fr. vol. 14, 377-466 (1989). A more modern and accurate account of the history of XAFS (EXAFS and XANES) is given by the leader of the group that developed the modern version of XAFS in the award lecture "Musings about the development of the XAFS" by Edward A. Stern, J. Synchrotron Rad. (2001). 8, 49-54.
References
Relevant Websites
- International XAFS Society
- FEFF Project, University of Washington, Seattle
- GNXAS project and XAS laboratory, Università di Camerino
- Community web site for XAFS
Books
- Scott Calvin, "XAFS for Everyone", CRC Press, 2013
- G. Bunker, "Introduction to XAFS: A Practical Guide to X-ray Absorption Fine Structure Spectroscopy", Cambridge University Press, 2010
- B.-K. Teo, EXAFS: basic principles and data analysis, Springer 1986
- X-ray Absorption: principles, applications and techniques of EXAFS, SEXAFS and XANES, a cura di D.C. Koeningsberger, R. Prins, Wiley 1988
Book Chapters
- Kelly, S.D., Hesterberg, D., and Ravel, B., Analysis of Soils and Minerals Using X-ray Absorption Spectroscopy in Methods of Soil Analysis, Part 5 -Mineralogical Methods, (A.L. Ulery and L.R. Drees, Eds.) p. 367. Soil Science Society of America, Madison, WI, USA, 2008.
Papers
- Edward A. Stern, "Musing about the development of XAFS", J. Synchrotron Rad. (2001). 8, 49-54
- J.J. Rehr and R.C. Albers, "Theoretical approaches to X-ray absorption fine structure", Reviews of Modern Physics 72 (2000), 621-654
- A. Filipponi, A. Di Cicco and C.R. Natoli, "X-ray absorption spectroscopy and n-body distribution functions in condensed matter", Physical Review B 52/21 (1995) 15122-15148
- F. de Groot, "High-resolution X-ray emission and X-ray absorption spectroscopy", Chemical Reviews 101 (2001) 1779-1808
- F.W. Lytle, "The EXAFS family tree: a personal history of the development of extended X-ray absorption fine structure",
- Dale E. Sayers, Edward A. Stern, and Farrel W. Lytle, New Technique for Investigating Noncrystalline Structures: Fourier Analysis of the Extended X-Ray—Absorption Fine Structure, Phys. Rev. Lett. 27, 1204–1207 (1971).
- A. Kodre, I. Arčon, Proceedings of 36th International Conference on Microelectronics, Devices and Materials, MIDEM, Postojna, Slovenia, October 28-20, (2000), p. 191-196