Chemistry:Tetronic acid: Difference between revisions
From HandWiki
(linkage) |
Importwiki (talk | contribs) (over-write) |
||
| Line 33: | Line 33: | ||
| BoilingPt = | | BoilingPt = | ||
| Solubility = | | Solubility = | ||
| MagSus = | | MagSus = −52.5·10<sup>−6</sup> cm<sup>3</sup>/mol | ||
}} | }} | ||
| Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| Line 45: | Line 45: | ||
It interconverts between keto and enol [[Chemistry:Tautomer|tautomer]]s:<ref>{{Cite journal | doi = 10.1016/j.arabjc.2015.11.004| title = Advancements in tetronic acid chemistry. Part 1: Synthesis and reactions| journal = Arabian Journal of Chemistry| year = 2015| last1 = Abdou| first1 = Moaz M.| last2 = El-Saeed| first2 = Rasha A.| last3 = Abozeid| first3 = Mohamed A.| last4 = Elattar| first4 = Khaled M.| last5 = Zaki| first5 = E.G.| last6 = Barakat| first6 = Y.| last7 = Ibrahim| first7 = V.| last8 = Fathy| first8 = Mahmoud| last9 = Amine| first9 = M.| last10 = Bondock| first10 = Samir| volume = 12| issue = 4| pages = 464–475| doi-access = free}}</ref> | It interconverts between keto and enol [[Chemistry:Tautomer|tautomer]]s:<ref>{{Cite journal | doi = 10.1016/j.arabjc.2015.11.004| title = Advancements in tetronic acid chemistry. Part 1: Synthesis and reactions| journal = Arabian Journal of Chemistry| year = 2015| last1 = Abdou| first1 = Moaz M.| last2 = El-Saeed| first2 = Rasha A.| last3 = Abozeid| first3 = Mohamed A.| last4 = Elattar| first4 = Khaled M.| last5 = Zaki| first5 = E.G.| last6 = Barakat| first6 = Y.| last7 = Ibrahim| first7 = V.| last8 = Fathy| first8 = Mahmoud| last9 = Amine| first9 = M.| last10 = Bondock| first10 = Samir| volume = 12| issue = 4| pages = 464–475| doi-access = free}}</ref> | ||
: | :250px | ||
Many [[Chemistry:Natural product|natural product]]s such as [[Chemistry:Ascorbic acid|ascorbic acid]] (vitamin C), [[Chemistry:Penicillic acid|penicillic acid]], [[Chemistry:Pulvinic acid|pulvinic acid]]s, and abyssomicins possess the β-keto-γ-butyrolactone motif of tetronic acid.<ref>{{Cite journal | doi = 10.1055/s-2006-950202| title = Synthetic Strategies towards Naturally Occurring Tetronic Acids| journal = Synthesis| volume = 2006| issue = 19| pages = 3157| year = 2006| last1 = Georgiadis| first1 = Dimitris| last2 = Zografos| first2 = Alexandros}}</ref> | Many [[Chemistry:Natural product|natural product]]s such as [[Chemistry:Ascorbic acid|ascorbic acid]] (vitamin C), [[Chemistry:Penicillic acid|penicillic acid]], [[Chemistry:Pulvinic acid|pulvinic acid]]s, and abyssomicins possess the β-keto-γ-butyrolactone motif of tetronic acid.<ref>{{Cite journal | doi = 10.1055/s-2006-950202| title = Synthetic Strategies towards Naturally Occurring Tetronic Acids| journal = Synthesis| volume = 2006| issue = 19| pages = 3157| year = 2006| last1 = Georgiadis| first1 = Dimitris| last2 = Zografos| first2 = Alexandros}}</ref> | ||
In [[Chemistry:Organic synthesis|organic synthesis]], it is used as a precursor for other substituted and ring-fused [[Chemistry:Furan|furan]]s and [[Chemistry:Butenolide|butenolide]]s.<ref>{{Cite web | url = https://www.alfa.com/en/catalog/A14079 | publisher = Alfa Aesar | title = Tetronic acid}}</ref><ref>{{cite journal | doi = 10.1021/jo00159a029| title = Substituted .gamma.-butyrolactones. Part 31. 2,4(3H,5H)-Furandione: Heteroannulations with aromatic o-amino carbonyl compounds and condensations with some vic-polyones| journal = The Journal of Organic Chemistry| volume = 48| issue = 11| pages = 1914| year = 1983| last1 = Schmidt| first1 = Diane Grob| last2 = Seemuth| first2 = Paul D.| last3 = Zimmer| first3 = Hans}}</ref> It is also forms the structural core of a class of pesticides, known as tetronic acid insecticides, which includes [[Chemistry:Spirodiclofen|spirodiclofen]] and spiromesifen. | In [[Chemistry:Organic synthesis|organic synthesis]], it is used as a precursor for other substituted and ring-fused [[Chemistry:Furan|furan]]s and [[Chemistry:Butenolide|butenolide]]s.<ref>{{Cite web | url = https://www.alfa.com/en/catalog/A14079 | publisher = Alfa Aesar | title = Tetronic acid}}</ref><ref>{{cite journal | doi = 10.1021/jo00159a029| title = Substituted .gamma.-butyrolactones. Part 31. 2,4(3H,5H)-Furandione: Heteroannulations with aromatic o-amino carbonyl compounds and condensations with some vic-polyones| journal = The Journal of Organic Chemistry| volume = 48| issue = 11| pages = 1914| year = 1983| last1 = Schmidt| first1 = Diane Grob| last2 = Seemuth| first2 = Paul D.| last3 = Zimmer| first3 = Hans}}</ref> It is also forms the structural core of a class of pesticides, known as tetronic acid insecticides, which includes [[Chemistry:Spirodiclofen|spirodiclofen]] and spiromesifen.<ref name=":0">{{Cite book |last=Jeschke |first=Peter |url=https://onlinelibrary.wiley.com/doi/book/10.1002/9783527699261 |title=Modern Crop Protection Compounds |last2=Witschel |first2=Matthias |last3=Krämer |first3=Wolfgang |last4=Schirmer |first4=Ulrich |date=25 January 2019 |publisher=Wiley‐VCH |isbn=9783527699261 |edition=3rd |pages=1202-1222 |chapter=32.4 Inhibitors of Lipid Synthesis: Acetyl‐CoA Carboxylase Inhibitors.}}</ref> | ||
==See also== | ==See also== | ||
Latest revision as of 01:36, 28 May 2025
| |
| Names | |
|---|---|
| Preferred IUPAC name
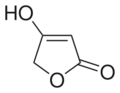
4-Hydroxyfuran-2(5H)-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| Properties | |
| C4H4O3 | |
| Molar mass | 100.073 g·mol−1 |
| Melting point | 141–143 °C (286–289 °F; 414–416 K) (dec.)[1] |
| −52.5·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Tetronic acid is a chemical compound, classified as a γ-lactone, with the molecular formula C4H4O3.
It interconverts between keto and enol tautomers:[2]
- 250px
Many natural products such as ascorbic acid (vitamin C), penicillic acid, pulvinic acids, and abyssomicins possess the β-keto-γ-butyrolactone motif of tetronic acid.[3]
In organic synthesis, it is used as a precursor for other substituted and ring-fused furans and butenolides.[4][5] It is also forms the structural core of a class of pesticides, known as tetronic acid insecticides, which includes spirodiclofen and spiromesifen.[6]
See also
References
- ↑ "2,4(3H,5H)-Furandione". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/254592?lang=en.
- ↑ Abdou, Moaz M.; El-Saeed, Rasha A.; Abozeid, Mohamed A.; Elattar, Khaled M.; Zaki, E.G.; Barakat, Y.; Ibrahim, V.; Fathy, Mahmoud et al. (2015). "Advancements in tetronic acid chemistry. Part 1: Synthesis and reactions". Arabian Journal of Chemistry 12 (4): 464–475. doi:10.1016/j.arabjc.2015.11.004.
- ↑ Georgiadis, Dimitris; Zografos, Alexandros (2006). "Synthetic Strategies towards Naturally Occurring Tetronic Acids". Synthesis 2006 (19): 3157. doi:10.1055/s-2006-950202.
- ↑ "Tetronic acid". Alfa Aesar. https://www.alfa.com/en/catalog/A14079.
- ↑ Schmidt, Diane Grob; Seemuth, Paul D.; Zimmer, Hans (1983). "Substituted .gamma.-butyrolactones. Part 31. 2,4(3H,5H)-Furandione: Heteroannulations with aromatic o-amino carbonyl compounds and condensations with some vic-polyones". The Journal of Organic Chemistry 48 (11): 1914. doi:10.1021/jo00159a029.
- ↑ Jeschke, Peter; Witschel, Matthias; Krämer, Wolfgang; Schirmer, Ulrich (25 January 2019). "32.4 Inhibitors of Lipid Synthesis: Acetyl‐CoA Carboxylase Inhibitors.". Modern Crop Protection Compounds (3rd ed.). Wiley‐VCH. pp. 1202-1222. ISBN 9783527699261. https://onlinelibrary.wiley.com/doi/book/10.1002/9783527699261.
 |

