Chemistry:5-Hydroxy-2(5H)-furanone
From HandWiki
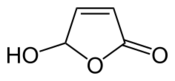
| |
| Names | |
|---|---|
| Preferred IUPAC name
5-Hydroxyfuran-2(5H)-one | |
| Other names
5-Hydroxy-5H-furan-2-one, 2-Hydroxyfuranone-(5), β-oxo-γ-butyrolactone, 4-Oxobut-2-enoic acid, 2,5-dihydrofuran-2-one, beta-Formylacrylic acid lactol, 2-Oxo-5-hydroxy-2,5-dihydrofuran, 2-Hydroxy-2,5-dihydro-5-furanone, 5-Hydroxy-2,5-dihydrofuran-2-one; γ-Hydroxybutenolide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4H4O3 | |
| Molar mass | 100.073 g·mol−1 |
| Density | 1.503 g/mL |
| Melting point | 55 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
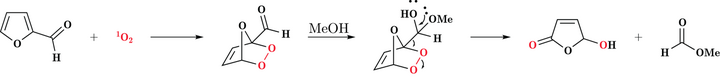
5-Hydroxy-2(5H)-furanone is a furanone derived from oxidation of furfural using singlet oxygen.[1][2] This oxidation is carried out generally in methanol or ethanol with a sensitizer like methylene blue or Rose bengal. The mechanism of this reaction is depicted as below.
Uses
5-Hydroxy-2(5H)-furanone is a potent pesticide[1] and a four carbon building block for various heterocycles.[3][4]
Chemical properties
5-Hydroxy-2(5H)-furanone exists in chemical equilibrium with its isomer, cis-β-formylacrylic acid, in ring-chain tautomerism:[5]
Under some conditions the compound will isomerize into succinic anhydride. Upon heating in strongly basic solution (pH > 9) this isomer will hydrate to succinic acid.[5]
See also
References
- ↑ 1.0 1.1 Hoydonckx, H. E.; Rhijn, W. M. Van; Rhijn, W. Van; De Vos, D. E.; Jacobs, P. A. (2007). "Furfural and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry: 5. doi:10.1002/14356007.a12_119.pub2. ISBN 978-3527306732.
- ↑ Zeitsch, K.J. (2000). The Chemistry and Technology of Furfural and its Many By-Products (Sugar Series) (1st ed.). Elsevier Science. pp. 170–171. ISBN 9780444503510. https://books.google.com/books?id=HWkCqDDlUt0C&dq=2-Hydroxyfuranone-%285%29+rose+bengal&pg=PA170.
- ↑ Fariña, Francisco; Martín, M. Rosario; Martín, Victoria; de Guereñu, Ana Martínez (1994). "Cycloaddition of Nitrile Oxides to 4-Oxobut-2-enoic Acid Derivatives". Heterocycles 38 (6): 1307–1316. doi:10.3987/COM-94-6679.
- ↑ L. Feringa, Ben; de Lange, Ben; Kok, Johan; S. Faber, Wijnand (1994). "Catalytic kinetic resolution of 5-alkoxy-2(5H)-furanones". Tetrahedron 50 (16): 4775–4794. doi:10.1016/S0040-4020(01)85016-X.
- ↑ 5.0 5.1 Poskonin, V. V.; Badovskaya, L. A. (2003). "Unusual Conversion of 5-Hydroxy-2(5H)furanone in Aqueous Solution". Chemistry of Heterocyclic Compounds 39 (5): 594–597. doi:10.1023/A:1025137914137.
 |