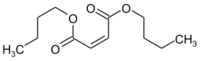
Chemistry:Maleic acid dibutyl ester
From HandWiki
Revision as of 06:16, 30 June 2021 by imported>Rtexter1 (simplify)
| |
| Names | |
|---|---|
| IUPAC name
Dibutyl (2Z)-2-butenedioate
| |
| Systematic IUPAC name
not | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| MeSH | maleate dibutyl maleate |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H20O4 | |
| Molar mass | 228.288 g·mol−1 |
| Appearance | Colorless to yellowish liquid with a characteristic odor[1] |
| Density | 0.99 g·cm−3[1] |
| Melting point | −85 °C (−121 °F; 188 K)[1] |
| Boiling point | 280 °C (536 °F; 553 K)[1] |
| Very hardly soluble (0.17 g·l−1 at 20 °C)[1] | |
| Vapor pressure | 0.0027 hPa (20 °C)[1] |
Refractive index (nD)
|
1.445 (20 °C)[2] |
| Hazards | |
| GHS pictograms |    [1] [1]
|
| H317, H373, H411[1] | |
| P273, P280, P302+352, P314[1] | |
| Flash point | 141 °C (286 °F; 414 K)[1] |
| 265 °C (509 °F; 538 K)[1] | |
| Explosive limits | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Maleic acid dibutyl ester is a chemical compound in the group of carboxylic acid esters.
Preparation
Maleic acid dibutyl ester can be prepared by the reaction of maleic acid anhydride and 1-butanol in presence of p-toluenesulfonic acid.[3][4]
Properties
Maleic acid dibutyl ester is a flammable, hard to ignite, oily, colorless to yellowish liquid with a characteristic odor, which has very low solubility in water.
Uses
Maleic acid dibutyl ester is mainly used as a plasticizer for aqueous dispersions of copolymers with vinyl acetate and as an intermediate in the preparation of other chemical compounds.[5]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 Record of Maleinsäuredibutylester in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 3 April 2019.
- ↑ Sigma-Aldrich Co., Dibutyl maleate, 96%. Retrieved on 2019-04-03.
- ↑ R. Wen, L. Long, L. Ding, Silas Yu (2001). "Study on synthesis of dibutyl maleate". Jishou Daxue Xuebao/Journal of Jishou University 22 (1): 78–80.
- ↑ B. Trivedi (2013). Maleic Anhydride. Springer Science & Business Media. pp. 277. ISBN 978-1-4757-0940-7. https://books.google.com/books?id=c8XeBwAAQBAJ&pg=PA277.
- ↑ (pdf) Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Dibutyl maletate (Report). OECD. http://www.inchem.org/documents/sids/sids/105760.pdf. Retrieved 3 April 2019.

