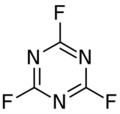
Chemistry:Cyanuric fluoride
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,4,6-trifluoro-1,3,5-triazine
| |||
| Other names
trifluorotriazine,
2,4,6-trifluoro-s-triazine, cyanuryl fluoride embox | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 3389 1935 | ||
| |||
| |||
| Properties | |||
| C3F3N3 | |||
| Molar mass | 135.047 g/mol | ||
| Appearance | colourless liquid | ||
| Density | 1.574 g/cm3 | ||
| Melting point | −38 °C (−36 °F; 235 K) | ||
| Boiling point | 74 °C (165 °F; 347 K) | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H310, H314, H330 | |||
| P260, P262, P264, P270, P271, P280, P284, P301+330+331, P302+350, P303+361+353, P304+340, P305+351+338, P310, P320, P321, P322, P361, P363, P403+233, P405, P501 | |||
| Related compounds | |||
Related compounds
|
cyanuric acid, cyanuric chloride, cyanuric bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Cyanuric fluoride or 2,4,6-trifluoro-1,3,5-triazine is a chemical compound with the formula (CNF)3. It is a colourless, pungent liquid. It has been used as a precursor for fibre-reactive dyes, as a specific reagent for tyrosine residues in enzymes, and as a fluorinating agent.[1]
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[2]
Preparation and reactions
Cyanuric fluoride is prepared by fluorinating cyanuric chloride. The fluorinating agent may be SbF3Cl2,[3] KSO2F,[4] or NaF.[5][6]
Cyanuric fluoride is used for the mild and direct conversion of carboxylic acids to acyl fluorides:[7]
Other fluorinating methods are less direct and may be incompatible with some functional groups.[8]
Cyanuric fluoride hydrolyses easily to cyanuric acid and it reacts more readily with nucleophiles than cyanuric chloride.[4] Pyrolysis of cyanuric fluoride at 1300 °C is a way to prepare cyanogen fluoride:[9]
- (CNF)3 → 3 CNF.
References
- ↑ "Fluorinated aromatic compounds". Kirk-Othmer Encyclopedia of Chemical Technology. 11. Wiley-Interscience. 1994. pp. 608.
- ↑ 40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities (July 1, 2008 ed.). Government Printing Office. http://edocket.access.gpo.gov/cfr_2008/julqtr/pdf/40cfr355AppA.pdf. Retrieved October 29, 2011.
- ↑ Abe F. Maxwell; John S. Fry; Lucius A. Bigelow (1958). "The Indirect Fluorination of Cyanuric Chloride". Journal of the American Chemical Society 80 (3): 548–549. doi:10.1021/ja01536a010.
- ↑ 4.0 4.1 Daniel W. Grisley, Jr; E. W. Gluesenkamp; S. Allen Heininger (1958). "Reactions of Nucleophilic Reagents with Cyanuric Fluoride and Cyanuric Chloride". Journal of Organic Chemistry 23 (11): 1802–1804. doi:10.1021/jo01105a620.
- ↑ C. W. Tullock; D. D. Coffman (1960). "Synthesis of Fluorides by Metathesis with Sodium Fluoride". Journal of Organic Chemistry 25 (11): 2016–2019. doi:10.1021/jo01081a050.
- ↑ Steffen Groß; Stephan Laabs; Andreas Scherrmann; Alexander Sudau; Nong Zhang; Udo Nubbemeyer (2000). "Improved Syntheses of Cyanuric Fluoride and Carboxylic Acid Fluorides". Journal für Praktische Chemie 342 (7): 711–714. doi:10.1002/1521-3897(200009)342:7<711::AID-PRAC711>3.0.CO;2-M.
- ↑ George A. Olah; Masatomo Nojima; Istvan Kerekes (1973). "Synthetic Methods and Reactions; IV. Fluorination of Carboxylic Acids with Cyanuric Fluoride". Synthesis 1973 (8): 487–488. doi:10.1055/s-1973-22238.
- ↑ Barda, David A. (2005). "Cyanuric Fluoride". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. pp. 77. doi:10.1002/047084289X.rn00043. ISBN 0471936235.
- ↑ F. S. Fawcett; R. D. Lipscomb (1964). "Cyanogen Fluoride: Synthesis and Properties". Journal of the American Chemical Society 86 (13): 2576–2579. doi:10.1021/ja01067a011.
 |