Chemistry:Propiolic acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Prop-2-ynoic acid[1] | |
| Other names
Propiolic acid
Acetylene carboxylic acid Propargylic acid Acetylene mono-carboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 878176 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 81893 | |
| KEGG | |
| MeSH | C011537 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H2O2 | |
| Molar mass | 70.047 g·mol−1 |
| Density | 1.1325 g/cm3 |
| Melting point | 9 °C (48 °F; 282 K) |
| Boiling point | 144 °C (291 °F; 417 K) (decomposes) |
| Acidity (pKa) | pka = 1.89 [2] |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H226, H301, H310, H315, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P262, P264, P270, P271, P280, P301+310, P302+350, P302+352, P303+361+353, P304+340, P310, P312, P321, P322, P330, P332+313, P361, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
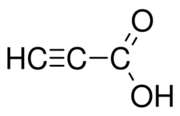
Propiolic acid is the organic compound with the formula HC2CO2H. It is the simplest acetylenic carboxylic acid. It is a colourless liquid that crystallises to give silky crystals. Near its boiling point, it decomposes.
It is soluble in water and possesses an odor like that of acetic acid.[4][5]
Preparation
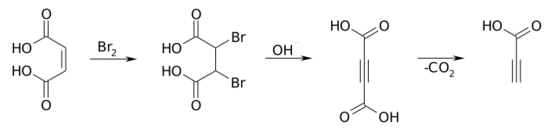
It is prepared commercially by oxidizing propargyl alcohol at a lead electrode.[6] It can also be prepared by decarboxylation of acetylenedicarboxylic acid.
Reactions and applications
Exposure to sunlight converts it into trimesic acid (benzene-1,3,5-tricarboxylic acid).[5] It undergoes bromination to give dibromoacrylic acid. With hydrogen chloride it forms chloroacrylic acid. Its ethyl ester condenses with hydrazine to form pyrazolone.[5]
It forms a characteristic explosive solid upon treatment to its aqueous solution with ammoniacal silver nitrate.[5] An amorphous explosive precipitate forms with ammoniacal cuprous chloride.
Propiolates
Propiolates are esters or salts of propiolic acid. Common examples include methyl propiolate and ethyl propiolate.
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 748. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ "Propiolic acid". https://pubchem.ncbi.nlm.nih.gov/compound/propiolic_acid.
- ↑ "Propiolic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/10110#section=Safety-and-Hazards.
- ↑ ed, Susan Budavari (1990). The Merck index an encyclopedia of chemicals, drugs, and biologicals (11. ed., 2. print. ed.). Rahway, NJ: Merck. pp. 7833, 1911. ISBN 9780911910285. https://archive.org/details/merckindexency00buda/page/7833.
- ↑ 5.0 5.1 5.2 5.3 Chisholm, Hugh, ed (1911). "Propiolic Acid". Encyclopædia Britannica. 22 (11th ed.). Cambridge University Press. p. 449.
- ↑ Wilhelm Riemenschneider (2002). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_235. ISBN 3527306730.
 |