Chemistry:Pyrazolone
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes.[1]
Structure and synthesis
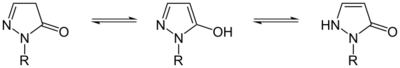
Pyrazolone can exist in two isomers: 3-pyrazolone and 4-pyrazolone.
These isomers can interconvert via lactam–lactim and imine–enamine tautomerism; these conversion often display photochromism. For pyrazolone derivatives, the 3-pyrazolone isomer can be stabilized with N-alkyl or N-aryl substituents.
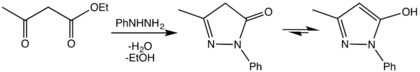
The first synthesis of pyrazolones was reported in 1883 by Ludwig Knorr, via a condensation reaction between ethyl acetoacetate and phenylhydrazine.[2]
Many pyrazolones are produced by functionalization of preformed pyrazolones.
Applications
Pharmaceuticals
This section is missing information about safety profile of analgesics (which ones are withdrawn and whether there is a common theme in adverse effects). (August 2022) |
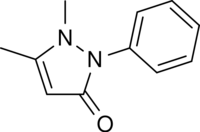
Pyrazolones are amongst the oldest synthetic pharmaceuticals, starting with the introduction of antipyrine (phenazone) in 1880s.[3][4] The compounds generally act as analgesics and include dipyrone (Metamizole), aminophenazone, ampyrone, famprofazone, morazone, nifenazone, piperylon and propyphenazone. Of these dipyrone is perhaps the most widely used.[3][5]
The basic structure has been also used in a number of newer drugs of other effects.[5] Edaravone is useful for prevention and/or therapy of arterial wall injury.[6] Eltrombopag is used to address low blood platelet count.
Dyes
Pyrazolone groups are present in several important dyes. They are commonly used in combination with azo groups to give a sub-family of azo dyes; sometimes referred to as azopyrazolones (tartrazine, orange B, mordant red 19, yellow 2G). Acid Yellow 17, Acid Yellow 23 (tartrazine), Pigment Yellow 13, and Pigment Red 38 are produced on the multi-ton scale annually.[1]
Ligands
Pyrazolones have been studied as ligands.[7]
References
- ↑ 1.0 1.1 Kornis, Gabe I. (2000). "Pyrazoles, Pyrazolines, and Pyrazolones". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.1625180111151814.a01. ISBN 0471238961.
- ↑ Knorr, Ludwig (July 1883). "Einwirkung von Acetessigester auf Phenylhydrazin" (in German). Berichte der Deutschen Chemischen Gesellschaft 16 (2): 2597–2599. doi:10.1002/cber.188301602194. https://zenodo.org/record/1425305.
- ↑ 3.0 3.1 Brogden, Rex N. (1986). "Pyrazolone Derivatives". Drugs 32 (Supplement 4): 60–70. doi:10.2165/00003495-198600324-00006. PMID 3552586.
- ↑ Brune, Kay (December 1997). "The early history of non-opioid analgesics". Acute Pain 1 (1): 33–40. doi:10.1016/S1366-0071(97)80033-2.
- ↑ 5.0 5.1 Zhao, Zefeng; Dai, Xufen; Li, Chenyang; Wang, Xiao; Tian, Jiale; Feng, Ying; Xie, Jing; Ma, Cong et al. (2020). "Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect". European Journal of Medicinal Chemistry 186: 111893. doi:10.1016/j.ejmech.2019.111893. PMID 31761383.
- ↑ Bailly, Christian; Hecquet, Paul-Emile; Kouach, Mostafa; Thuru, Xavier; Goossens, Jean-François (2020). "Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone". Bioorganic & Medicinal Chemistry 28 (10): 115463. doi:10.1016/j.bmc.2020.115463. PMID 32241621.
- ↑ CASAS, J; GARCIATASENDE, M; SANCHEZ, A; SORDO, J; TOUCEDA, A (June 2007). "Coordination modes of 5-pyrazolones: A solid-state overview". Coordination Chemistry Reviews 251 (11–12): 1561–1589. doi:10.1016/j.ccr.2007.02.010.
External links
- Pyrazolones at the US National Library of Medicine Medical Subject Headings (MeSH)
- Pubchem - 3-Pyrazolone
- Pubchem - 4-Pyrazolone
 |