Chemistry:Acyl cyanide
From HandWiki
Short description: Chemical group (–C(O)C≡N)
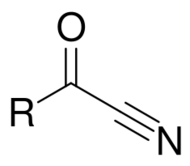
In organic chemistry, an acyl cyanide is a functional group with the formula R–C(O)CN and structure R–C(=O)–C≡N. It consists of an acyl group (R–C=O) attached to cyanide (–C≡N). Examples include acetyl cyanide, formyl cyanide, and oxalyl dicyanide. Acyl cyanides are reagents in organic synthesis.[1][2]
Synthesis
Classically acyl cyanides are produced by the salt metathesis reaction of acyl chlorides with sodium cyanide:
- [math]\displaystyle{ {\color{red}\ce{R-C(O)}}\ce{Cl} + \ce{Na}{\color{red}\ce{CN}} \longrightarrow {\color{red}\ce{R-C(O)CN}} + \ce{NaCl} }[/math]
Alternatively, they can be produced by dehydration of acyl aldoximes:
- [math]\displaystyle{ {\color{red}\ce{R-C(O)C}}\ce{H=}{\color{red}\ce{N}}\ce{OH} \longrightarrow {\color{red}\ce{R-C(O)CN}} + \ce{H2O} }[/math]
Acetyl cyanide is also prepared by hydrocyanation of ketene:
- [math]\displaystyle{ \ce{CH2=}{\color{red}\ce{C=O}} + \ce{H}{\color{red}\ce{CN}} \longrightarrow \ce{H3C -}{\color{red}\ce{C(O)CN}} }[/math]
Reactions
They are mild acylating agents.[2] With aqueous base, acyl cyanides break down to cyanide and the carboxylate:[3]
- [math]\displaystyle{ {\color{red}\ce{R-C(O)CN}} + \ce{2 NaOH} \longrightarrow {\color{red}\ce{R-CO}}\ce{_2Na} + \ce{Na}{\color{red}\ce{CN}} + \ce{H2O} }[/math]
With azides, acyl cyanides undergo the click reaction to give acyl tetrazoles.[4]
References
- ↑ Liu, Bing; Wang, Yong; Chen, Ying; Wu, Qian; Zhao, Jing; Sun, Jianwei (2018). "Stereoselective Synthesis of Fully-Substituted Acrylonitriles via Formal Acylcyanation of Electron-Rich Alkynes". Organic Letters 20 (12): 3465–3468. doi:10.1021/acs.orglett.8b01180. PMID 29873500.
- ↑ 2.0 2.1 Morris, Joel (2001). "Acetyl Cyanide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ra026. ISBN 0471936235.
- ↑ Hünig, Siegfried; Schaller, Rainer (1982). "The Chemistry of Acyl Cyanides". Angewandte Chemie International Edition in English 21: 36–49. doi:10.1002/anie.198200361.
- ↑ Demko, Zachary P.; Sharpless, K. Barry (2002). "A Click Chemistry Approach to Tetrazoles by Huisgen 1,3-Dipolar Cycloaddition: Synthesis of 5-Acyltetrazoles from Azides and Acyl Cyanides". Angewandte Chemie International Edition 41 (12): 2113–2116. doi:10.1002/1521-3773(20020617)41:12<2113::AID-ANIE2113>3.0.CO;2-Q. PMID 19746613.
 |