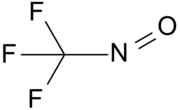
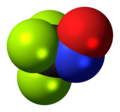
Chemistry:Trifluoronitrosomethane
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trifluoro(nitroso)methane | |||
| Other names
Trifluoro-nitrosomethane
Trifluoro-nitroso-methane Nitrosotrifluoromethane | |||
| Identifiers | |||
| Abbreviations | TFNM | ||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| Properties | |||
| CF3NO | |||
| Molar mass | 99.012 g·mol−1 | ||
| Appearance | Deep blue gas[1] Purple solid | ||
| Melting point | −196.6 °C (−321.9 °F; 76.5 K) | ||
| Boiling point | −85 °C (−121 °F; 188 K) | ||
| Hazards | |||
| Main hazards | Toxic | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trifluoronitrosomethane (commonly abbreviated TFNM) is a toxic organic compound consisting of a trifluoromethyl group covalently bound to a nitroso group. Its distinctive deep blue color is unusual for a gas.
History
Trifluoronitrosomethane was synthesized for the first time in 1936 by Otto Ruff and Manfred Giese at the University of Wrocław.[2] It was created through the fluorination of silver cyanide in the presence of silver nitrate and silver oxide.
Production
Trifluoronitrosomethane can be produced from the reaction of trifluoroiodomethane and nitric oxide under a UV light with a yield of up to 90% in normal pressure. A small amount of mercury is needed as catalyst. The reaction results in the creation of iodine as a by-product.[3][4][5]
Properties
Although it is somewhat more kinetically stable due to its fluorine substituents, trifluoronitrosomethane, like other nitroso compounds, has a weak C–N bond of only 39.9 kcal/mol.[6]
Related
Trifluoronitrosoethylene is also a similar deep blue gas.[7]
See also
References
- ↑ Griffin, C. E.; Haszeldine, R. N. (1960). "279. Perfluoroalkyl derivatives of nitrogen. Part VIII. Trifluoronitrosoethylene and its polymers". Journal of the Chemical Society (Resumed): 1398. doi:10.1039/JR9600001398.
- ↑ Ruff, Otto; Giese, Manfred (1936). "Das Trifluor-nitroso-methan, CF3.NO (III.)". Ber Dtsch Chem Ges 69 (4): 684–689. doi:10.1002/cber.19360690411.
- ↑ Senning, Alexander (1964). "N-, 0-, and S-trihalomethyl compounds". Chemical Reviews 65 (4): 385–412. doi:10.1021/cr60236a001.
- ↑ Taylor, C. W.; Brice, T. J.; Wear, R. L. (1962). "The Preparation of Polyfluoronitrosoalkanes from Nitrosyl Polyfluoroacylates". Journal of Organic Chemistry 27 (3): 1064–1066. doi:10.1021/jo01050a523.
- ↑ Park, J. D.; Rosser, R. W.; Lacher, J. R. (1962). "Preparation of Perfluoronitrosoalkanes. Reaction of Trifluoroacetic Anhydride with Nitrosyl Chloride". Journal of Organic Chemistry 27 (4): 1642. doi:10.1021/jo01051a519.
- ↑ Luo, Yu-Ran (2007). Comprehensive Handbook of Chemical Bond Energies. Boca Raton, Fl.: CRC Press. pp. 406. ISBN 978-0-8493-7366-4.
- ↑ Griffin, C. E.; Haszeldine, R. N. (1960). "279. Perfluoroalkyl derivatives of nitrogen. Part VIII. Trifluoronitrosoethylene and its polymers". Journal of the Chemical Society (Resumed): 1398–1406. doi:10.1039/JR9600001398.
External links
 |