Physics:Optogenetic methods to record cellular activity
Optogenetics began with methods to alter neuronal activity with light, using e.g. channelrhodopsins. In a broader sense, optogenetic approaches also include the use of genetically encoded biosensors to monitor the activity of neurons or other cell types by measuring fluorescence or bioluminescence. Genetically encoded calcium indicators (GECIs) are used frequently to monitor neuronal activity, but other cellular parameters such as membrane voltage or second messenger activity can also be recorded optically. The use of optogenetic sensors is not restricted to neuroscience, but plays increasingly important roles in immunology, cardiology and cancer research.
History
The first experiments to measure intracellular calcium levels via protein expression were based on aequorin, a bioluminescent protein from the jellyfish Aequorea. To produce light, however, this enzyme needs the 'fuel' compound coelenteracine, which has to be added to the preparation. This is not practical in intact animals, and in addition, the temporal resolution of bioluminescence imaging is relatively poor (seconds-minutes). The first genetically encoded fluorescent calcium indicator (GECI) to be used to image activity in an animal was cameleon, designed by Atsushi Miyawaki, Roger Tsien and coworkers in 1997.[1] Cameleon was first used successfully in an animal by Rex Kerr, William Schafer and coworkers to record from neurons and muscle cells of the nematode C. elegans.[2] Cameleon was subsequently used to record neural activity in flies[3] and zebrafish.[4] In mammals, the first GECI to be used in vivo was GCaMP,[5] first developed by Junichi Nakai and coworkers in 2001.[6] GCaMP has undergone numerous improvements, notably by a team of scientists at the Janelia Farm Research Campus (GENIE project, HHMI), and GCaMP6[7] in particular has become widely used in neuroscience. Very recently, G protein-coupled receptors have been harnessed to generate a series of highly specific indicators for various neurotransmitters.[8][9]
Design principles
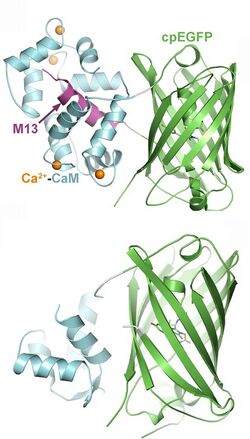
Genetically encoded sensors are fusion proteins, consisting of a ligand binding domain (sensor) and a fluorescent protein, attached by a short linker (flexible peptide). When the sensor domain binds the correct ligand, it changes conformation. This movement is transferred to the fluorescent protein and the resulting deformation leads to a change in fluorescence. The efficiency of this process depends critically on the length of the linker region, which has to be optimized in a labor-intensive process. The fluorescent protein is often circularly permuted, i.e. new C-terminal and N-terminal ends were created. Single-wavelength sensors are easy to use for qualitative measurements, but difficult to calibrate for quantitative measurements of ligand concentration.
A second class of sensors relies on Förster resonance energy transfer (FRET) between two fluorescent proteins (FP) of different color. The shorter wavelength FP (donor) is excited with blue light from a laser or LED. If the second FP (acceptor) is very close, the energy is transferred to the acceptor, resulting in yellow or red fluorescence. When the acceptor FP moves further away, the donor emits green fluorescence. The sensor domain is typically spliced between the two FPs, resulting in a hinge-type movement upon ligand binding that changes the distance between donor and acceptor. The imaging procedure is more complex for FRET sensors, but the fluorescence ratio can be calibrated to measure the absolute concentration of a ligand. Read-out via fluorescence lifetime imaging (FLIM) of donor fluorescence is also possible, as the FRET process speeds up the fluorescence decay.
Advantages of optogenetic sensors
- can be targeted to specific classes of cells (e.g. astrocytes or pyramidal cells). This allows for optical read-out without spatial resolution, e.g. fiber photometry from deep brain areas.[10]
- can be targeted to sub-cellular compartments (e.g. synapses, organelles, nucleus) by fusing the indicator protein with specific anchoring domains, retention signals or intrabodies.
- work in a variety of species (nematodes, insects, fish, mammals) and in cell culture systems (FLIPR assay)
- can be delivered by viral vectors (e.g. rAAV)
- can be used to record the activity of thousands of neurons at the same time [11]
Drawbacks, limitations
- will buffer the measured ion or protein, potentially interfering with cellular signaling
- are subject to photobleaching, compromising long-term measurements
- can be toxic when expressed at very high concentration
- require highly sensitive cameras or laser scanning microscopes
- some GPCR-based sensors are sensitive to polarization[12]
- most indicators are green fluorescent, making it difficult to measure several cellular parameters simultaneously (multiplexing).
Classes of genetically encoded indicators
Indicators have been designed to measure ion concentrations, membrane potential, neurotransmitters, and various intracellular signaling molecules. The following list provides only examples for each class; many more have been published.
Intracellular signaling
- Genetically encoded calcium indicators (GECI): A large class of tools, based on natural calcium binding proteins (calmodulin, troponin). Different affinities, kinetics, and colors (green, red) available. Read-out via fluorescence intensity (single wavelength indicators), FRET or BRET. Have been targeted to various organelles. Current version: JGCaMP8[13]
- Genetically encoded chloride indicators: Clomeleon[14]
- Genetically encoded potassium indicators: GINKO2[15]
- Genetically encoded indicators for intracellular pH (GEPhI): CypHer[16]
- Genetically encoded voltage indicators (GEVI): ArcLight[17]
- Genetically encoded vesicle fusion sensors: Synapto-pHluorin, Synaptophysin-pHluorin[18]
- Genetically encoded cAMP sensors: EPAC[19]
- Genetically encoded ATP sensors: QUEEN-37C[20]
- Genetically encoded kinase activity sensors: CaMui,[21] SmURFP
- Genetically encoded Small G-protein sensors: FRas[22]
Neurotransmitters and other extracellular signals
- Genetically encoded glutamate sensors: GluSnFR[23]
- Genetically encoded GABA sensors: iGABASnFR[24]
- Genetically encoded dopamine sensors: dLight1,[25] GRAB-DA[26]
- Genetically encoded serotonin sensors: GRAB5-HT,[27] sDarken[28]
- Genetically encoded norepinephrine sensors: GRABNE[29]
- Genetically encoded sensor for endocannabinoid activity: GRABeCB2.0[30]
- Genetically encoded sensor for orexin/hypocretin neuropeptides: OxLight1[31]
- Genetically encoded sensor for lactate: eLACCO1.1[32]
Further reading
A recent review of GPCR-based genetically encoded fluorescent indicators for neuromodulators [9]
External links
- Fluorescent Biosensor Database, a fairly complete searchable list of published sensors and their basic properties, maintained by Jin Zhang's lab at UCSD.[33]
- Fluorescent Biosensors available on Addgene, a nonprofit plasmid repository.
References
- ↑ "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature 388 (6645): 882–887. August 1997. doi:10.1038/42264. PMID 9278050. Bibcode: 1997Natur.388..882M.
- ↑ "Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans". Neuron 26 (3): 583–594. June 2000. doi:10.1016/s0896-6273(00)81196-4. PMID 10896155.
- ↑ "Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons". Current Biology 12 (21): 1877–1884. October 2002. doi:10.1016/s0960-9822(02)01239-3. PMID 12419190.
- ↑ "Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator". Journal of Neurophysiology 90 (6): 3986–3997. December 2003. doi:10.1152/jn.00576.2003. PMID 12930818.
- ↑ "Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle". The Journal of Biological Chemistry 279 (20): 21461–21468. May 2004. doi:10.1074/jbc.M401084200. PMID 14990564.
- ↑ "A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein". Nature Biotechnology 19 (2): 137–141. February 2001. doi:10.1038/84397. PMID 11175727.
- ↑ "Ultrasensitive fluorescent proteins for imaging neuronal activity". Nature 499 (7458): 295–300. July 2013. doi:10.1038/nature12354. PMID 23868258. Bibcode: 2013Natur.499..295C.
- ↑ "A Bright and Colorful Future for G-Protein Coupled Receptor Sensors". Frontiers in Cellular Neuroscience 14: 67. 2020. doi:10.3389/fncel.2020.00067. PMID 32265667.
- ↑ 9.0 9.1 Rohner, Valentin Lu; Lamothe‐Molina, Paul J.; Patriarchi, Tommaso (2024-01-30). "Engineering, applications, and future perspectives of GPCR ‐based genetically encoded fluorescent indicators for neuromodulators" (in en). Journal of Neurochemistry. doi:10.1111/jnc.16045. ISSN 0022-3042. https://onlinelibrary.wiley.com/doi/10.1111/jnc.16045.
- ↑ "Dopamine D1 receptor signalling in dyskinetic Parkinsonian rats revealed by fiber photometry using FRET-based biosensors". Scientific Reports 10 (1): 14426. September 2020. doi:10.1038/s41598-020-71121-8. PMID 32879346. Bibcode: 2020NatSR..1014426J.
- ↑ "Q&A: The brain under a mesoscope: the forest and the trees". BMC Biology 15 (1): 82. September 2017. doi:10.1186/s12915-017-0426-y. PMID 28911321.
- ↑ "Orthogonally-polarized excitation for improved two-photon and second-harmonic-generation microscopy, applied to neurotransmitter imaging with GPCR-based sensors" (in EN). Biomedical Optics Express 13 (2): 777–790. February 2022. doi:10.1364/BOE.448760. PMID 35284188.
- ↑ "jGCaMP8 Fast Genetically Encoded Calcium Indicators". Janelia Research Campus: 361685. 2020. doi:10.25378/JANELIA.13148243.
- ↑ "Imaging synaptic inhibition throughout the brain via genetically targeted Clomeleon". Brain Cell Biology 36 (1–4): 101–118. August 2008. doi:10.1007/s11068-008-9031-x. PMID 18850274.
- ↑ Wu, Sheng-Yi; Wen, Yurong; Serre, Nelson B. C.; Laursen, Cathrine Charlotte Heiede; Dietz, Andrea Grostøl; Taylor, Brian R.; Drobizhev, Mikhail; Molina, Rosana S. et al. (2022-09-06). Dutzler, Raimund. ed. "A sensitive and specific genetically-encoded potassium ion biosensor for in vivo applications across the tree of life" (in en). PLOS Biology 20 (9): e3001772. doi:10.1371/journal.pbio.3001772. ISSN 1545-7885. PMID 36067248.
- ↑ "Fluorescent indicators for intracellular pH". Chemical Reviews 110 (5): 2709–2728. May 2010. doi:10.1021/cr900249z. PMID 19831417.
- ↑ "Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe". Neuron 75 (5): 779–785. September 2012. doi:10.1016/j.neuron.2012.06.040. PMID 22958819.
- ↑ "Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses". Neuron 51 (6): 773–786. September 2006. doi:10.1016/j.neuron.2006.08.029. PMID 16982422.
- ↑ "Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity". PLOS ONE 10 (4): e0122513. 2015-04-14. doi:10.1371/journal.pone.0122513. PMID 25875503. Bibcode: 2015PLoSO..1022513K.
- ↑ "Live cell imaging of metabolic heterogeneity by quantitative fluorescent ATP indicator protein, QUEEN-37C" (in en). bioRxiv: 2021.10.08.463131. 2021-10-09. doi:10.1101/2021.10.08.463131.
- ↑ "Activation of CaMKII in single dendritic spines during long-term potentiation". Nature 458 (7236): 299–304. March 2009. doi:10.1038/nature07842. PMID 19295602. Bibcode: 2009Natur.458..299L.
- ↑ "An improved Ras sensor for highly sensitive and quantitative FRET-FLIM imaging". PLOS ONE 8 (1): e52874. 2013-01-14. doi:10.1371/journal.pone.0052874. PMID 23349692. Bibcode: 2013PLoSO...852874O.
- ↑ "Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR". Nature Methods 15 (11): 936–939. November 2018. doi:10.1038/s41592-018-0171-3. PMID 30377363.
- ↑ "A genetically encoded fluorescent sensor for in vivo imaging of GABA". Nature Methods 16 (8): 763–770. August 2019. doi:10.1038/s41592-019-0471-2. PMID 31308547.
- ↑ "Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors". Science 360 (6396): eaat4422. June 2018. doi:10.1126/science.aat4422. PMID 29853555.
- ↑ "GPCR-Based Dopamine Sensors-A Detailed Guide to Inform Sensor Choice for In vivo Imaging". International Journal of Molecular Sciences 21 (21): 8048. October 2020. doi:10.3390/ijms21218048. PMID 33126757.
- ↑ "A genetically encoded sensor for measuring serotonin dynamics". Nature Neuroscience 24 (5): 746–752. May 2021. doi:10.1038/s41593-021-00823-7. PMID 33821000.
- ↑ Kubitschke, Martin; Müller, Monika; Wallhorn, Lutz; Pulin, Mauro; Mittag, Manuel; Pollok, Stefan; Ziebarth, Tim; Bremshey, Svenja et al. (2022-12-06). "Next generation genetically encoded fluorescent sensors for serotonin" (in en). Nature Communications 13 (1): 7525. doi:10.1038/s41467-022-35200-w. ISSN 2041-1723. PMID 36473867.
- ↑ "A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine". Neuron 102 (4): 745–761.e8. May 2019. doi:10.1016/j.neuron.2019.02.037. PMID 30922875.
- ↑ "A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo". Nature Biotechnology 40 (5): 787–798. November 2021. doi:10.1038/s41587-021-01074-4. PMID 34764491.
- ↑ "A genetically encoded sensor for in vivo imaging of orexin neuropeptides". Nature Methods 19 (2): 231–241. February 2022. doi:10.1038/s41592-021-01390-2. PMID 35145320.
- ↑ Nasu, Yusuke; Murphy-Royal, Ciaran; Wen, Yurong; Haidey, Jordan N.; Molina, Rosana S.; Aggarwal, Abhi; Zhang, Shuce; Kamijo, Yuki et al. (2021-12-06). "A genetically encoded fluorescent biosensor for extracellular l-lactate" (in en). Nature Communications 12 (1): 7058. doi:10.1038/s41467-021-27332-2. ISSN 2041-1723. PMID 34873165.
- ↑ "Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks". Chemical Reviews 118 (24): 11707–11794. December 2018. doi:10.1021/acs.chemrev.8b00333. PMID 30550275.
 |