Chemistry:Daprodustat
 | |
| Clinical data | |
|---|---|
| Trade names | Duvroq, Jesduvroq |
| Other names | GSK1278863 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a623010 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Hypoxia-inducible factor prolyl hydroxylase inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
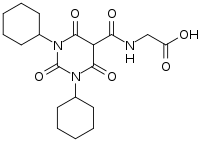
| Formula | C19H27N3O6 |
| Molar mass | 393.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Daprodustat, sold under the brand name Duvroq among others, is a medication that is used for the treatment of anemia due to chronic kidney disease.[1] It is a hypoxia-inducible factor prolyl hydroxylase inhibitor.[1] It is taken by mouth.[1]
The most common side effects include high blood pressure, thrombotic vascular events, abdominal pain, dizziness and allergic reactions.[2]
Daprodustat was approved for medical use in Japan in June 2020,[3][4] and in the United States in February 2023.,[1][2][5] making it the first oral treatment for anemia caused by chronic kidney disease for adults in the US.[2] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[6]
Medical uses
Daprodustat is indicated for the treatment of anemia due to chronic kidney disease.[1]
History
Daprodustat increases erythropoietin levels.[2] The effectiveness of daprodustat was established in a randomized study of 2,964 adult participants receiving dialysis.[2] In this study, participants received either oral daprodustat or injected recombinant human erythropoietin (a standard of care treatment for people with anemia due to chronic kidney disease).[2] Daprodustat raised and maintained the hemoglobin (the protein in red blood cells that carries oxygen and is a common measure of anemia) within the target range of 10-11 grams/deciliter, similar to that of the recombinant human erythropoietin.[2] The US Food and Drug Administration (FDA) granted the approval of Jesduvroq to GlaxoSmithKline LLC.[2]
Society and culture
Due to its potential applications in athletic doping, it has also been incorporated into screens for performance-enhancing drugs.[7]
Research
Daprodustat is in phase III clinical trials for the treatment of anemia caused by chronic kidney disease.[8][9][10]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 "Jesduvroq- daprodustat tablet, film coated". 1 February 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d82aa06e-5a33-4844-99b7-4701313455a4.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 "FDA Approves First Oral Treatment for Anemia Caused by Chronic Kidney Disease for Adults on Dialysis". U.S. Food and Drug Administration (FDA) (Press release). 1 February 2023. Archived from the original on 4 February 2023. Retrieved 3 February 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Daprodustat: First Approval". Drugs 80 (14): 1491–1497. September 2020. doi:10.1007/s40265-020-01384-y. PMID 32880805.
- ↑ "GSK receives first regulatory approval for Duvroq (daprodustat) in Japan for patients with anaemia due to chronic kidney disease" (Press release). GSK. 29 June 2020. Archived from the original on 4 February 2023. Retrieved 29 March 2021.
- ↑ "FDA Approves First Oral Treatment for Kidney Disease–Induced Anemia". JAMA 329 (9): 704. 15 February 2023. doi:10.1001/jama.2023.1556. PMID 36790833. https://jamanetwork.com/journals/jama/fullarticle/2801721.
- ↑ (PDF) New Drug Therapy Approvals 2023 (Report). January 2024. https://www.fda.gov/media/175253/download. Retrieved 9 January 2024.
- ↑ "Mass spectrometric characterization of a prolyl hydroxylase inhibitor GSK1278863, its bishydroxylated metabolite, and its implementation into routine doping controls". Drug Testing and Analysis 8 (8): 858–63. August 2016. doi:10.1002/dta.1870. PMID 26361079.
- ↑ "Investigational therapies for renal disease-induced anemia". Expert Opinion on Investigational Drugs 25 (8): 901–16. August 2016. doi:10.1080/13543784.2016.1182981. PMID 27122198.
- ↑ "Discovery and Preclinical Characterization of GSK1278863 (Daprodustat), a Small Molecule Hypoxia Inducible Factor-Prolyl Hydroxylase Inhibitor for Anemia". The Journal of Pharmacology and Experimental Therapeutics 363 (3): 336–347. December 2017. doi:10.1124/jpet.117.242503. PMID 28928122.
- ↑ "Daprodustat for the Treatment of Anemia in Patients Not Undergoing Dialysis". The New England Journal of Medicine 385 (25): 2313–2324. December 2021. doi:10.1056/NEJMoa2113380. PMID 34739196.
External links
- Clinical trial number NCT02879305 for "Anemia Studies in Chronic Kidney Disease: Erythropoiesis Via a Novel Prolyl Hydroxylase Inhibitor Daprodustat-Dialysis (ASCEND-D)" at ClinicalTrials.gov
 |

