Chemistry:Iron(II) carbonate
| |
| Names | |
|---|---|
| Other names
ferrous carbonate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| FeCO3 | |
| Molar mass | 115.854 g/mol |
| Appearance | white powder or crystals |
| Density | 3.9 g/cm3[1] |
| Melting point | decomposes |
| 0.0067 g/L;[2] Ksp = 1.28 × 10−11 [3] | |
Solubility product (Ksp)
|
3.13×10−11[4] |
| +11,300·10−6 cm3/mol | |
| Structure | |
| Hexagonal scalenohedral / Trigonal (32/m) Space group: R 3c, a = 4.6916 Å, c = 15.3796 Å | |
| 6 | |
| Related compounds | |
Other anions
|
iron(II) sulfate |
Other cations
|
copper(II) carbonate, zinc carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
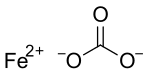
Iron(II) carbonate, or ferrous carbonate, is a chemical compound with formula FeCO3, that occurs naturally as the mineral siderite. At ordinary ambient temperatures, it is a green-brown ionic solid consisting of iron(II) cations Fe2+ and carbonate anions CO2−3.[5]
Preparation
Ferrous carbonate can be prepared by reacting solution of the two ions, such as iron(II) chloride and sodium carbonate:[5]
- FeCl2 + Na2CO3 → FeCO3 + 2NaCl
Ferrous carbonate can be prepared also from solutions of an iron(II) salt, such as iron(II) perchlorate, with sodium bicarbonate, releasing carbon dioxide:[6]
- Fe(ClO4)2 + 2NaHCO3 → FeCO3 + 2NaClO4 + CO2 + H2O
Sel and others used this reaction (but with FeCl2 instead of Fe(ClO4)2) at 0.2 M to prepare amorphous FeCO3.[7]
Care must be taken to exclude oxygen O2 from the solutions, because the Fe2+ ion is easily oxidized to Fe3+, especially at pH above 6.0.[6]
Ferrous carbonate also forms directly on steel or iron surfaces exposed to solutions of carbon dioxide, forming an "iron carbonate" scale:[3]
- Fe + CO2 + H2O → FeCO3 + H2
Properties
The dependency of the solubility in water with temperature was determined by Wei Sun and others to be
- [math]\displaystyle{ \log K_{\mathit{sp}} = -59.3498 - 0.041377 T - 2.1963/T + 24.5724 \log T + 2.518 \sqrt{I} - 0.657 I, }[/math]
where T is the absolute temperature in kelvins, and I is the ionic strength of the liquid.[3]
Iron carbonate decomposes at about 500–600 °C (773–873 K).[8]
Uses
Ferrous carbonate has been used as an iron dietary supplement to treat anemia.[9] It is noted to have very poor bioavailability in cats and dogs.[10]
Toxicity
Ferrous carbonate is slightly toxic; the probable oral lethal dose is between 0.5 and 5 g/kg (between 35 and 350 g for a 70 kg person).[11]
Iron(III) carbonate
Unlike iron(II) carbonate, iron(III) carbonate has not been isolated. Attempts to produce iron(III) carbonate by the reaction of aqueous ferric ions and carbonate ions result in the production of iron(III) oxide with the release of carbon dioxide or bicarbonate.[12]
References
- ↑ D R. Lide, ed.(2000): "CRC Handbook of Chemistry and Physics". 81st Edition. Pages 4-65.
- ↑ Patty, F., ed. (1963): "Industrial Hygiene and Toxicology"; volume II: 'Toxicology". 2nd ed. Interscience. Page 1053.
- ↑ 3.0 3.1 3.2 Wei Sun (2009): "Kinetics of iron carbonate and iron sulfide scale formation in CO2/H2S corrosion". PhD Thesis, Ohio University.
- ↑ John Rumble (June 18, 2018) (in English). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–188. ISBN 1138561630.
- ↑ 5.0 5.1 (1995): "Kirk-Othmer Encyclopedia of Chemical Technology". 4th ed. Volume 1.
- ↑ 6.0 6.1 Philip C. Singer and Werner Stumm (1970): "The solubility of ferrous iron in carbonate-bearing waters". Journal of the American Water Works Association, volume 62, issue 3, pages 198-202. https://www.jstor.org/stable/41266171
- ↑ Ozlem Sel, A.V. Radha, Knud Dideriksen, and Alexandra Navrotsky (2012): "Amorphous iron (II) carbonate: Crystallization energetics and comparison to other carbonate minerals related to CO2 sequestration". Geochimica et Cosmochimica Acta, volume 87, issue 15, pages 61–68. doi:10.1016/j.gca.2012.03.011
- ↑ "Kinetics of Thermal Decomposition of Iron Carbonate". Egyptian Journal of Chemistry 53 (6): 871–884. 2010-12-31. doi:10.21608/ejchem.2010.1268. ISSN 2357-0245. http://dx.doi.org/10.21608/ejchem.2010.1268.
- ↑ A .Osol and J. E. Hoover and others, eds. (1975): "Remington's Pharmaceutical Sciences". 15th ed. Mack Publishing. Page 775
- ↑ "AAFCO methods for substantiating nutritional adequacy of dog and cat foods (proposed for 2014 publication)". AAFCO. 2013. https://www.aafco.org/Portals/0/SiteContent/Regulatory/Committees/Pet-Food/Reports/Pet_Food_Report_2013_Midyear-Proposed_Revisions_to_AAFCO_Nutrient_Profiles.pdf.
- ↑ Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-97
- ↑ Ronald Rich (2007). "8 Iron through Hassium" (in en). Inorganic Reactions in Water (1st ed.). Springer Berlin, Heidelberg. p. 178. ISBN 9783540739616. https://doi.org/10.1007/978-3-540-73962-3.
| H2CO3 | He | ||||||||||||||||
| Li2CO3, LiHCO3 |
BeCO3 | B | C | (NH4)2CO3, NH4HCO3 |
O | F | Ne | ||||||||||
| Na2CO3, NaHCO3, Na3H(CO3)2 |
MgCO3, Mg(HCO3)2 |
Al2(CO3)3 | Si | P | S | Cl | Ar | ||||||||||
| K2CO3, KHCO3 |
CaCO3, Ca(HCO3)2 |
Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe |
| Cs2CO3, CsHCO3 |
BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | (BiO)2CO3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La2(CO3)3 | Ce2(CO3)3 | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UO2CO3 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |

