Chemistry:Phenazopyridine
 | |
| Clinical data | |
|---|---|
| Trade names | Pyridium |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682231 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
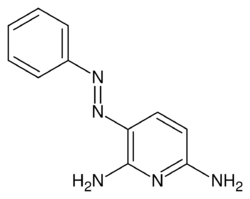
| Formula | C11H11N5 |
| Molar mass | 213.244 g·mol−1 |
| | |
Phenazopyridine is a medication which, when excreted by the kidneys into the urine, has a local analgesic effect on the urinary tract. It is often used to help with the pain, irritation, or urgency caused by urinary tract infections, surgery, or injury to the urinary tract.
In 2021, it was the 285th most commonly prescribed medication in the United States, with more than 700,000 prescriptions.[1][2]
Medical uses
Phenazopyridine is prescribed for its local analgesic effects on the urinary tract. It is sometimes used in conjunction with an antibiotic or other anti-infective medication at the beginning of treatment to help provide immediate symptomatic relief. Phenazopyridine does not treat infections or injury; it is only used for symptom relief.[3][4] It is recommended that it be used for no longer than the first two days of antibacterial treatment as longer treatment may mask symptoms.[4]
Phenazopyridine is also prescribed for other cases requiring relief from irritation or discomfort during urination. For example, it is often prescribed after the use of an in-dwelling Foley catheter, endoscopic (cystoscopy) procedures, or after urethral, prostate, or urinary bladder surgery which may result in irritation of the epithelial lining of the urinary tract.[3]
This medication is not used to treat infection and may mask symptoms of inappropriately treated UTI. It provides symptom relief during a UTI, following surgery, or injury to the urinary tract. UTI therapy should be limited to 1–2 days.[4] Long-term use of phenazopyridine can mask symptoms.[5]
Side effects
Phenazopyridine produces a vivid color change in urine, typically to a dark orange to reddish color. This effect is common and harmless, and indeed a key indicator of the presence of the medication in the body. Users of phenazopyridine are warned not to wear contact lenses, as phenazopyridine has been known to permanently discolor contact lenses and fabrics.[3][6] It also tends to leave an orange-yellow stain on surfaces it comes in contact with. Some may be mistakenly concerned that this indicated blood in the urine.
Phenazopyridine can also cause headaches, upset stomach (especially when not taken with food), or dizziness. Less frequently it can cause a pigment change in the skin or eyes, to a noticeable yellowish color. This is due to a depressed excretion via the kidneys causing a buildup of the medication in the skin, and normally indicates a need to discontinue usage.[4] Other such side effects include fever, confusion, shortness of breath, skin rash, and swelling of the face, fingers, feet, or legs.[3][4] Long-term use may cause yellowing of nails.[7]
Phenazopyridine should be avoided by people with glucose-6-phosphate dehydrogenase deficiency,[4][8][9][10] because it can cause hemolysis (destruction of red blood cells) due to oxidative stress.[11] It has been reported to cause methemoglobinemia after overdose and even normal doses.[12] In at least one case the patient had pre-existing low levels of methemoglobin reductase,[13] which likely predisposed her to the condition. It has also been reported to cause sulfhemoglobinemia.[4][14][15] [16]
Phenazopyridine is an azo dye.[17][18] Other azo dyes, which were previously used in textiles, printing, and plastic manufacturing, have been implicated as carcinogens that can cause bladder cancer.[19] While phenazopyridine has never been shown to cause cancer in humans, evidence from animal models suggests that it is potentially carcinogenic.[4][20]
Pregnancy
This medication has shown no adverse events in animal models, but no human trials have been conducted.[4] It is not known if phenazopyridine is excreted in breast milk.[4]
Pharmacokinetics
The full pharmacokinetic properties of phenazopyridine have not been determined. It has mostly been studied in animal models, but they may not be very representative of humans.[21] Rat models have shown its half-life to be 7.35 hours,[22] and 40% is metabolized hepatically (by the liver).[22]
Mechanism of action
Phenazopyridine's mechanism of action is not well known, and only basic information on its interaction with the body is available. It is known that the chemical has a direct topical analgesic effect on the mucosa lining of the urinary tract. It is rapidly excreted by the kidneys directly into the urine.[21] Hydroxylation is the major form of metabolism in humans,[21] and the azo bond is usually not cleaved.[21] On the order of 65% of an oral dose will be secreted directly into the urine chemically unchanged.[4]
Brand names
In addition to its generic form, phenazopyridine is distributed under the following brand names:
References
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Phenazopyridine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Phenazopyridine.
- ↑ 3.0 3.1 3.2 3.3 "Pyridium Plus Tablets". http://www.wcrx.com/pdfs/pi/pi_pyridium_plus.pdf.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 "PYRIDIUM (phenazopyridine) tablet, film coated". http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae0f0808-51ed-43b9-86a3-b947c42da89f.
- ↑ "Urinary tract infections". British Medical Journal 2 (5648): 687–706. April 1969. doi:10.1016/j.pop.2013.06.005. PMID 5776230.
- ↑ "Phenazopyridine: MedlinePlus Drug Information" (in en). American Society of Health-System Pharmacists, Inc.. https://medlineplus.gov/druginfo/meds/a682231.html.
- ↑ "Lemon-yellow nails and long-term phenazopyridine use". Annals of Internal Medicine 127 (12): 1137. December 1997. doi:10.7326/0003-4819-127-12-199712150-00040. PMID 9412335.
- ↑ "Phenazopyridine-induced hemolytic anemia in a patient with G6PD deficiency". Acta Haematologica 70 (3): 208–209. 1983. doi:10.1159/000206727. PMID 6410650.
- ↑ "Phenazopyridine-induced hemolytic anemia in G-6-PD deficiency". Drug Intelligence & Clinical Pharmacy 21 (11): 921–922. November 1987. doi:10.1177/106002808702101116. PMID 3678069.
- ↑ "Acute hemolytic anaemia due to phenazopyridine hydrochloride in G-6-PD deficient subject". Lancet 2 (8297): 564. September 1982. doi:10.1016/s0140-6736(82)90651-1. PMID 6125724.
- ↑ "Diagnosis and management of G6PD deficiency". American Family Physician 72 (7): 1277–1282. October 2005. PMID 16225031. http://www.aafp.org/afp/20051001/1277.html.
- ↑ "Acquired methemoglobinemia and hemolytic anemia after usual doses of phenazopyridine". Drug Intelligence & Clinical Pharmacy 16 (2): 157–159. February 1982. doi:10.1177/106002808201600212. PMID 7075467.
- ↑ "Phenazopyridine induced methaemoglobinaemia associated with decreased activity of erythrocyte cytochrome b5 reductase". Journal of Medical Genetics 20 (4): 307–309. August 1983. doi:10.1136/jmg.20.4.307. PMID 6620333.
- ↑ "Phenazopyridine-induced sulfhemoglobinemia: inadvertent rechallenge". The American Journal of Medicine 91 (3): 315–317. September 1991. doi:10.1016/0002-9343(91)90135-K. PMID 1892154.
- ↑ "Acrocyanosis from phenazopyridine-induced sulfhemoglobinemia mistaken for Raynaud phenomenon". Journal of Clinical Rheumatology 15 (3): 127–129. April 2009. doi:10.1097/RHU.0b013e31819db6db. PMID 19300288.
- ↑ "Phenazopyridine-induced sulfhemoglobinemia". The Annals of Pharmacotherapy 39 (6): 1128–1130. June 2005. doi:10.1345/aph.1E557. PMID 15886294.
- ↑ Cystitis in Females~treatment at eMedicine
- ↑ "Phenazopyridine Hydrochloride". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/phenazopyridine-hydrochloride.html.
- ↑ Transitional Cell Carcinoma Imaging at eMedicine
- ↑ "Phenazopyridine Hydrochloride". National Toxicology Program. https://ntp.niehs.nih.gov/ntp/roc/content/profiles/phenazopyridinehydrochloride.pdf.
- ↑ 21.0 21.1 21.2 21.3 "Excretion of phenazopyridine and its metabolites in the urine of humans, rats, mice, and guinea pigs". Journal of Pharmaceutical Sciences 79 (4): 321–325. April 1990. doi:10.1002/jps.2600790410. PMID 2352143.
- ↑ 22.0 22.1 "Metabolism of phenazopyridine by isolated rat hepatocytes". Biopharmaceutics & Drug Disposition 14 (2): 171–179. March 1993. doi:10.1002/bdd.2510140208. PMID 8453026.
 |


