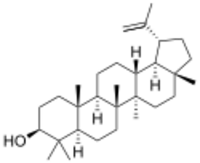
Chemistry:Lupeol
| |
| Names | |
|---|---|
| IUPAC name
(1R,3aR,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a,5a,5b,8,8,11a-hexamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysen-9-ol
| |
| Other names
(3β,13ξ)-Lup-20(29)-en-3-ol; Clerodol; Monogynol B; Fagarasterol; Farganasterol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H50O | |
| Molar mass | 426.729 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lupeol is a pharmacologically active pentacyclic triterpenoid. It has several potential medicinal properties, like anticancer and anti-inflammatory activity.[1]
Natural occurrences
Lupeol is found in a variety of plants, including mango, Acacia visco and Abronia villosa.[2] It is also found in dandelion coffee. Lupeol is present as a major component in Camellia japonica leaf.[1]
Total synthesis
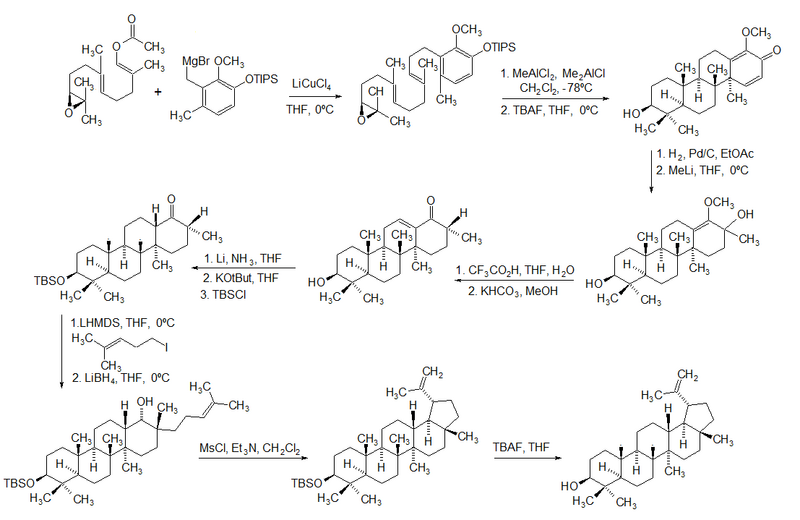
The first total synthesis of lupeol was reported by Gilbert Stork et al.[3]
In 2009, Surendra and Corey reported a more efficient and enantioselective total synthesis of lupeol, starting from (1E,5E)-8-[(2S)-3,3-dimethyloxiran-2-yl]-2,6-dimethylocta-1,5-dienyl acetate by use of a polycyclization.[4]
Biosynthesis
Lupeol is produced by several organisms from squalene epoxide. Dammarane and baccharane skeletons are formed as intermediates. The reactions are catalyzed by the enzyme lupeol synthase.[5] A recent study on the metabolomics of Camellia japonica leaf revealed that lupeol is produced from squalene epoxide where squalene play the role as a precursor.[1]
Pharmacology
Lupeol has a complex pharmacology, displaying antiprotozoal, antimicrobial, antiinflammatory, antitumor and chemopreventive properties.[6]
Animal models suggest lupeol may act as an anti-inflammatory agent. A 1998 study found lupeol to decrease paw swelling in rats by 39%, compared to 35% for the standardized control compound indomethacin.[7]
One study has also found some activity as a Dipeptidyl peptidase-4 inhibitor and prolyl oligopeptidase inhibitor at high concentrations (in the millimolar range).[8]
It is an effective inhibitor in laboratory models of prostate and skin cancers.[9][10][11]
As an anti-inflammatory agent, lupeol functions primarily on the interleukin system. Lupeol to decreases IL-4 (interleukin 4) production by T-helper type 2 cells.[6][12]
Lupeol has been found to have a contraceptive effect due to its inhibiting effect on the calcium channel of sperm (CatSper).[13]
Lupeol has also been shown to exert anti-angiogenic and anti-cancer effects via the downregulation of TNF-alpha and VEGFR-2.[14]
Famous anti-inflammatory ethno-medicinal plant Camellia japonica contains anti-inflammatory component lupeol in its leaf.[1]
See also
References
- ↑ 1.0 1.1 1.2 1.3 Majumder, Soumya; Ghosh, Arindam; Bhattacharya, Malay (2020-08-27). "Natural anti-inflammatory terpenoids in Camellia japonica leaf and probable biosynthesis pathways of the metabolome". Bulletin of the National Research Centre 44 (1): 141. doi:10.1186/s42269-020-00397-7. ISSN 2522-8307.
- ↑ "Abronione, a rotenoid from the desert annual Abronia villosa". Phytochemistry Letters 4 (2): 72–74. June 2011. doi:10.1016/j.phytol.2010.08.004. PMID 21617767.
- ↑ "Total synthesis of lupeol". Journal of the American Chemical Society 93 (19): 4945. 1971. doi:10.1021/ja00748a068.
- ↑ "A short enantioselective total synthesis of the fundamental pentacyclic triterpene lupeol". Journal of the American Chemical Society 131 (39): 13928–9. October 2009. doi:10.1021/ja906335u. PMID 19788328.
- ↑ "Solanum lycopersicum lupeol biosynthesis". http://solcyc.solgenomics.net/LYCO/NEW-IMAGE?type=PATHWAY&object=PWY-112.
- ↑ 6.0 6.1 Margareth B. C. Gallo; Miranda J. Sarachine (2009). "Biological activities of Lupeol". International Journal of Biomedical and Pharmaceutical Sciences 3 (Special Issue 1): 46–66. http://www.globalsciencebooks.info/JournalsSup/images/0906/IJBPS_3%28SI1%2946-66o.pdf.
- ↑ "Anti-inflammatory activity of lupeol and lupeol linoleate in rats". Journal of Ethnopharmacology 76 (1): 77–80. June 2001. doi:10.1016/S0378-8741(01)00175-1. PMID 11378285.
- ↑ "Flavonoids with prolyl oligopeptidase inhibitory activity isolated from Scutellaria racemosa Pers". Fitoterapia 81 (6): 552–6. September 2010. doi:10.1016/j.fitote.2010.01.018. PMID 20117183.
- ↑ "Protective effects of lupeol and mango extract against androgen induced oxidative stress in Swiss albino mice". Asian Journal of Andrology 10 (2): 313–8. March 2008. doi:10.1111/j.1745-7262.2008.00313.x. PMID 18097535.
- ↑ "Preventive effects of lupeol on DMBA induced DNA alkylation damage in mouse skin". Food and Chemical Toxicology 45 (11): 2331–5. November 2007. doi:10.1016/j.fct.2007.06.002. PMID 17637493.
- ↑ "Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice". Oncogene 23 (30): 5203–14. July 2004. doi:10.1038/sj.onc.1207641. PMID 15122342.
- ↑ "Suppression of T lymphocyte activity by lupeol isolated from Crataeva religiosa". Phytotherapy Research 20 (4): 279–87. April 2006. doi:10.1002/ptr.1852. PMID 16557610.
- ↑ "Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids". Proceedings of the National Academy of Sciences of the United States of America 114 (22): 5743–5748. May 2017. doi:10.1073/pnas.1700367114. PMID 28507119.
- ↑ "Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α". PLOS ONE 12 (12): e0189628. 2017-12-12. doi:10.1371/journal.pone.0189628. PMID 29232409. Bibcode: 2017PLoSO..1289628K.
 |