Biology:Dipeptidyl peptidase-4 inhibitor
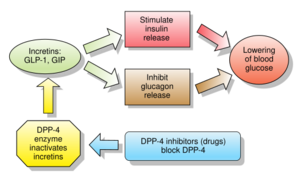
Inhibitors of dipeptidyl peptidase 4 (DPP-4 inhibitors or gliptins) are a class of oral hypoglycemics that block the enzyme dipeptidyl peptidase-4 (DPP-4). They can be used to treat diabetes mellitus type 2.
The first agent of the class – sitagliptin – was approved by the FDA in 2006.[1]
Glucagon increases blood glucose levels, and DPP-4 inhibitors reduce glucagon and blood glucose levels. The mechanism of DPP-4 inhibitors is to increase incretin levels (GLP-1 and GIP),[2][3][4] which inhibit glucagon release, which in turn increases insulin secretion, decreases gastric emptying, and decreases blood glucose levels.
A 2018 meta-analysis found no favorable effect of DPP-4 inhibitors on all-cause mortality, cardiovascular mortality, myocardial infarction or stroke in patients with type 2 diabetes.[5]
Examples
Drugs belonging to this class are:
- Sitagliptin[6] (FDA approved 2006, marketed by Merck & Co. as Januvia)
- Vildagliptin[7] (EU approved 2007, marketed in the EU by Novartis as Galvus)
- Saxagliptin (FDA approved in 2009, marketed as Onglyza)
- Linagliptin (FDA approved in 2011, marketed as Tradjenta by Eli Lilly and Company and Boehringer Ingelheim)[8]
- Gemigliptin (approved in Korea in 2012, marketed by LG Life Sciences)[9] Marketed as Zemiglo
- Anagliptin (approved in Japan as Suiny in 2012, marketed by Sanwa Kagaku Kenkyusho Co., Ltd. and Kowa Company, Ltd.)[10]
- Teneligliptin (approved in Japan as Tenelia in 2012[11])
- Alogliptin (FDA approved 2013 as Nesina/ Vipidia, marketed by Takeda Pharmaceutical Company)
- Trelagliptin (approved for use in Japan as Zafatek/ Wedica in 2015)
- Omarigliptin (MK-3102) (approved as Marizev in Japan in 2015,[12] developed by Merck & Co.; research showed that omarigliptin can be used as once-weekly treatment and generally well tolerated throughout the base and extension studies[13])
- Evogliptin (approved as Suganon/ Evodine for use in South Korea[14])
- Gosogliptin (approved as Saterex for use in Russia[15])
- Dutogliptin (PHX- 1149 free base, being developed by Phenomix Corporation), Phase III[16]
- Neogliptin[17]
- Retagliptin (SP-2086), approved in China.
- Denagliptin
- Cofrogliptin (HSK- 7653, compound 2)
- Fotagliptin
- Prusogliptin
Other chemicals which may inhibit DPP-4 include:
- Berberine, an alkaloid found in plants of the genus Berberis, inhibits dipeptidyl peptidase-4 which may at least partly explains its antihyperglycemic activity.[18]
Adverse effects
In those already taking sulphonylureas, there is an increased risk of low blood sugar when taking a medicine in the DPP-4 drug class.[19]
Adverse effects include nasopharyngitis, headache, nausea, heart failure, hypersensitivity and skin reactions.
The U.S. Food and Drug Administration (FDA) is warning that the type 2 diabetes medicines like sitagliptin, saxagliptin, linagliptin, and alogliptin may cause joint pain that can be severe and disabling. FDA has added a new Warning and Precaution about this risk to the labels of all medicines in this drug class, called dipeptidyl peptidase-4 (DPP-4) inhibitors.[20] However, studies assessing risk of rheumatoid arthritis among DPP-4 inhibitor users have been inconclusive.[21]
A 2014 review found increased risk of heart failure with saxagliptin and alogliptin, prompting the FDA in 2016 to add warnings to the relevant drug labels.[22]
A 2018 meta analysis showed that use of DPP-4 inhibitors was associated with a 58% increased risk of developing acute pancreatitis compared with placebo or no treatment.[23]
A 2018 observational study suggested an elevated risk of developing inflammatory bowel disease (specifically, ulcerative colitis), reaching a peak after three to four years of use and decreasing after more than four years of use.[24]
A 2020 Cochrane systematic review did not find enough evidence of reduction of all-cause mortality, serious adverse events, cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke or end-stage renal disease when comparing metformin monotherapy to dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes.[25]
Cancer
In response to a report of precancerous changes in the pancreases of rats and organ donors treated with the DPP-4 inhibitor sitagliptin,[26][27] the United States FDA and the European Medicines Agency each undertook independent reviews of all clinical and preclinical data related to the possible association of DPP-4 inhibitors with pancreatic cancer. In a joint letter to the New England Journal of Medicine, the agencies stated that they had not yet reached a final conclusion regarding a possible causative relationship.[28]
A 2014 meta-analysis found no evidence for increased pancreatic cancer risk in people treated with DPP-4 inhibitors, but owing to the modest amount of data available, was not able to completely exclude possible risk.[29]
Combination drugs
Some DPP-4 inhibitor drugs have received approval from the FDA to be used with metformin concomitantly with additive effect to increase the level of glucagon-like peptide 1 (GLP-1) which also decreases hepatic glucose production.[citation needed]
See also
- Development of dipeptidyl peptidase-4 inhibitors
References
- ↑ "FDA Approves New Treatment for Diabetes" (Press release). U.S. Food and Drug Administration. October 17, 2006. Retrieved 2006-10-17.
- ↑ "Dipeptidyl peptidase IV inhibitors: how do they work as new antidiabetic agents?". Regulatory Peptides 128 (2): 159–65. June 2005. doi:10.1016/j.regpep.2004.06.001. PMID 15780435.
- ↑ "Glucagon-like peptide 1 improved glycemic control in type 1 diabetes". BMC Endocrine Disorders 3 (1): 3. April 2003. doi:10.1186/1472-6823-3-3. PMID 12697069.
- ↑ "Glucagon-like peptide I reduces postprandial glycemic excursions in IDDM". Diabetes 44 (6): 626–30. June 1995. doi:10.2337/diabetes.44.6.626. PMID 7789625.
- ↑ "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis". JAMA 319 (15): 1580–1591. April 2018. doi:10.1001/jama.2018.3024. PMID 29677303.
- ↑ Banting and Best Diabetes Centre at UT sitagliptin
- ↑ Banting and Best Diabetes Centre at UT vildagliptin
- ↑ "FDA approves new treatment for Type 2 diabetes". Fda.gov. 2011-05-02. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm253501.htm.
- ↑ "LG Life Science". Lgls.com. http://www.lgls.com/rd/pipeline.jsp.
- ↑ "New Drugs Approved in FY 2012". http://www.pmda.go.jp/english/service/pdf/list/NewdrugsFY2012.pdf.
- ↑ Teneligliptin (Antidiabetic). To Market, To Market. 48. 2012. 523–524. doi:10.1016/b978-0-12-417150-3.00028-4. ISBN 9780124171503.
- ↑ "Merck MARIZEV Once-Weekly DPP-4 Inhibitor For Type2 Diabetes Approved In Japan". NASDAQ. 28 September 2015. http://www.nasdaq.com/article/merck-marizevonceweekly-dpp4-inhibitor-for-type2-diabetes-approved-in-japan-20150928-00333.
- ↑ "Safety and Efficacy of Omarigliptin (MK-3102), a Novel Once-Weekly DPP-4 Inhibitor for the Treatment of Patients With Type 2 Diabetes". Diabetes Care 38 (11): 2106–14. November 2015. doi:10.2337/dc15-0109. PMID 26310692.
- ↑ "Dong-A ST's DPP4 inhibitor, SUGANON, got approved for type 2 diabetes in Korea". pipelinereview.com. October 2, 2015. https://www.pipelinereview.com/index.php/2015100259148/Small-Molecules/Dong-A-STs-DPP4-inhibitor-SUGANON-got-approved-for-type-2-diabetes-in-Korea.html.
- ↑ "SatRx LLC Announces First Registration in Russia of SatRx (gosogliptin), an Innovative Drug for Treatment of Type 2 Diabetes" (Press release). SatRx LLC.
- ↑ "Forest Splits With Phenomix", San Diego Business Journal, Tuesday, April 20, 2010 http://www.sdbj.com/news/2010/apr/20/forest-splits-phenomix/
- ↑ "Design, Synthesis and Biological Evaluation of Neogliptin, a Novel 2-Azabicyclo[2.2.1heptane-Based Inhibitor of Dipeptidyl Peptidase-4 (DPP-4)"]. Pharmaceuticals 15 (3): 273. February 2022. doi:10.3390/ph15030273. PMID 35337071.
- ↑ "Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine". Journal of Enzyme Inhibition and Medicinal Chemistry 24 (5): 1061–6. October 2009. doi:10.1080/14756360802610761. PMID 19640223.
- ↑ "Addition of dipeptidyl peptidase-4 inhibitors to sulphonylureas and risk of hypoglycaemia: systematic review and meta-analysis". BMJ 353: i2231. May 2016. doi:10.1136/bmj.i2231. PMID 27142267.
- ↑ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". 2015-08-28. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm460238.htm.
- ↑ "DPP-4 Inhibitor-Induced Rheumatoid Arthritis Among Diabetics: A Nested Case-Control Study". Diabetes Therapy 9 (1): 141–151. February 2018. doi:10.1007/s13300-017-0353-5. PMID 29236221.
- ↑ "Diabetes Meds Containing Saxagliptin and Alogliptin Linked to Increased HF". Pharmacy Practice News. April 2016. http://www.pharmacypracticenews.com/Policy/Article/04-16/Diabetes-Meds-Containing-Saxagliptin-and-Alogliptin-Linked-to-Increased-HF/35964/ses=ogst.
- ↑ "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes: A Systematic Review and Meta-analysis". JAMA 319 (15): 1580–1591. April 2018. doi:10.1001/jama.2018.3024. PMID 29677303.
- ↑ "Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study". BMJ 360: k872. March 2018. doi:10.1136/bmj.k872. PMID 29563098.
- ↑ "Metformin monotherapy for adults with type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews 2020 (6): CD012906. June 2020. doi:10.1002/14651858.CD012906.pub2. PMID 32501595.
- ↑ "Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin". Diabetes 58 (7): 1604–15. July 2009. doi:10.2337/db09-0058. PMID 19403868.
- ↑ "Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors". Diabetes 62 (7): 2595–604. July 2013. doi:10.2337/db12-1686. PMID 23524641.
- ↑ "Pancreatic safety of incretin-based drugs--FDA and EMA assessment". The New England Journal of Medicine 370 (9): 794–7. February 2014. doi:10.1056/NEJMp1314078. PMID 24571751.
- ↑ "Dipeptidyl peptidase-4 inhibitors and pancreatitis risk: a meta-analysis of randomized clinical trials". Diabetes, Obesity & Metabolism 16 (1): 48–56. January 2014. doi:10.1111/dom.12176. PMID 23837679.
 |

