Chemistry:Cyclopentanone
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclopentanone | |||
| Other names
Ketocyclopentane
Adipic ketone | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C5H8O | |||
| Molar mass | 84.12 g/mol | ||
| Appearance | clear, colorless liquid | ||
| Odor | peppermint-like | ||
| Density | 0.95 g/cm3, liquid | ||
| Melting point | −58.2 °C (−72.8 °F; 215.0 K) | ||
| Boiling point | 130.6 °C (267.1 °F; 403.8 K) | ||
| Slightly soluble | |||
| -51.63·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet | Cyclopentanone | ||
| GHS pictograms |  
| ||
| GHS Signal word | WARNING | ||
| H226, H315, H319 | |||
| P210, P302+352, P305+351+338[2] | |||
| Flash point | 26 °C (79 °F; 299 K) | ||
| Related compounds | |||
Related ketones
|
cyclohexanone 2-pentanone 3-pentanone cyclopentenone | ||
Related compounds
|
cyclopropane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
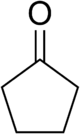
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopentanone:[3]
- (CH2)4(CO2H)2 → (CH2)4CO + H2O + CO2
Uses
Cyclopentanone is common precursor to fragrances, especially those related to jasmine and jasmone. Examples include 2-pentyl- and 2-heptylcyclopentanone.[4] It is a versatile synthetic intermediate, being a precursor to cyclopentobarbital.[5]
Cyclopentanone is also used to make cyclopentamine, the pesticide pencycuron, and pentethylcyclanone.[5]
It is also used as a precursor to cubane-1,4-dicarboxylate, which is used to synthesize other substituted cubanes, such as the high explosives heptanitrocubane and octonitrocubane.[6]
References
- ↑ Merck Index, 11th Edition, 2748.
- ↑ Sigma-Aldrich Co., Cyclopentanone.
- ↑ J. F. Thorpe and G. A. R. Kon (1925). "Cyclopentanone". Organic Syntheses 5: 37. http://www.orgsyn.org/demo.aspx?prep=CV1P0192.; Collective Volume, 1, pp. 192.
- ↑ Johannes Panten and Horst Surburg "Flavors and Fragrances, 2. Aliphatic Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2015, Wiley-VCH, Weinheim.doi:10.1002/14356007.t11_t01
- ↑ 5.0 5.1 Hardo Siegel; Manfred Eggersdorfer (2005). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077. ISBN 978-3-527-30673-2.
- ↑ Bliese, Marianne; Tsanaktsidis, John (1997). "Dimethyl Cubane-1,4-dicarboxylate: A Practical Laboratory Scale Synthesis" (in en). Australian Journal of Chemistry 50 (3): 189. doi:10.1071/C97021. ISSN 0004-9425. http://www.publish.csiro.au/?paper=C97021.
 |