Chemistry:Pentakis(dimethylamido)tantalum
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Tantalum(V) dimethylazanide
| |
| Other names
Pentakis(dimethylamino)tantalum(V), Tantalum dimethylamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C10H30N5Ta | |
| Molar mass | 401.333 g·mol−1 |
| Appearance | orange powder (xtl) |
| Melting point | 100 °C |
| Hazards | |
| GHS pictograms |  
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentakis(dimethylamido)tantalum is an organometallic compound of tantalum. It is a colorless solid that is soluble in organic solvents. It hydrolyzes readily to release dimethylamine.
Synthesis and structure
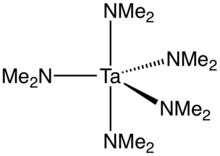
Ta(NMe2)5 is prepared by treating TaCl5 with lithium dimethylamide.[1] The preparation is similar to that for tetrakis(dimethylamido)titanium.
The compound has idealized D3h symmetry (ignoring the organic substituents). File:Tantalumamidocomplexes.tif
Applications to organic synthesis
The complex effects C-alkylation of secondary amines with 1-alkenes [2] and hydroaminoalkylation of olefins to form alkylamines.[3]
File:Hydroaminoalkylation hartwig correction.tif
References
- ↑ Bradley, D. C.; Thomas, I. M. (1962-07-01). "Metallo-Organic Compounds Containing Metal–Nitrogen Bonds: Part Iii. Dialkylamino Compounds of Tantalum". Canadian Journal of Chemistry 40 (7): 1355–1360. doi:10.1139/v62-207. ISSN 0008-4042.
- ↑ Clerici, Mario G.; Maspero, Federico (1980-01-01). "CatalyticC-Alkylation of Secondary Amines with Alkenes" (in en). Synthesis 1980 (4): 305–306. doi:10.1055/s-1980-29002. ISSN 0039-7881.
- ↑ Herzon, Seth B.; Hartwig, John F. (2007-05-01). "Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines". Journal of the American Chemical Society 129 (21): 6690–6691. doi:10.1021/ja0718366. ISSN 0002-7863. PMID 17474747.
 |

