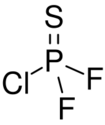
Chemistry:Phosphorothioic chloride difluoride
| |
| |
| Names | |
|---|---|
| IUPAC name
Phosphorothioic chloride difluoride
| |
| Systematic IUPAC name
Difluorochloro(sulfanylidene)-λ5-phosphane | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| |
| Properties | |
| PSClF 2 | |
| Molar mass | 136.48 g·mol−1 |
| Appearance | Colorless gas or liquid |
| Density | 5.579 g/L as gas |
| Melting point | −155.2 °C (−247.4 °F; 118.0 K) |
| Boiling point | 6.3 °C (43.3 °F; 279.4 K) |
| Structure | |
| Tetrahedral at the P atom | |
| Hazards | |
| Main hazards | toxic fumes |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphorothioic chloride difluoride or thiophosphoryl chloride difluoride is a chemical compound with formula PSClF
2. It is normally found as a gas boiling at 6.3 °C and melting at −155.2 °C. The density of the gas at standard conditions is 5.579 g/L.[1] Critical pressure is 41.4 bars, and critical temperature is 439.2 K.[2]
Production
Phosphorothioic chloride difluoride was made in 1940 by reacting PSCl
3 with SbF
3 and SbCl
5 at 75 °C.[3]
In another reaction PSCl
3 reacts with KSO
2F to make PSF
3, KCl and SO
2, but also partly yields PSClF
2.[4]
A small percentage of is formed when F
2P(=S)–S–P(–CF
3)
2 or (F
3C–)
2P(=S)–S–PF
2 reacts with chlorine.[5]
It can be formed from difluoro(germylthio)phosphine:
Properties
Although phosphorothioic chloride difluoride does not spontaneously ignite in air, mixtures with air are explosive. The gas is hydrolysed slowly by water vapour. It also reacts with potassium hydroxide solution.[3]
Heat of vaporization is 5703 cal/mol.[3]
Infrared bands in the gas are at 946, 920, 738, 541, 395, 361, 317, missed, and 198 cm−1. In liquid, a Raman spectroscopy has bands at 939, 913, 727, 536, 394, 359, 314, 251, 207. These are for PF
2 symmetric stretch in a' and a'' symmetry, PS stretch, PCl stretch, PCl bend, PF
2 scissor, PF
2 rock, PS op-bend, and PS ip-bend.[7][8]
The nuclear magnetic resonance coupling constants for 31
P are 1220. It is a triplet line with intensities 1:2:1. The chemical shift from orthophosphoric acid is −50×10−6. For 19
F the coupling constant is 1218. It is a doublet line in ratio 1:1. The chemical shift from CCl
3F is −15.9×10−6.[9]
References
- ↑ Haynes, William M. (2016-04-19) (in en). CRC Handbook of Chemistry and Physics, 94th Edition. CRC Press. ISBN 9781466571150. https://books.google.com/books?id=MmDOBQAAQBAJ&pg=SA4-PA81.
- ↑ CRC Handbook of Chemistry and Physics. pp. 6–39.
- ↑ 3.0 3.1 3.2 Advances in Inorganic Chemistry and Radiochemistry, Volume 2. Academic Press. 1960. ISBN 9780080578514. https://books.google.com/books?id=KxuDjYsMLxMC&pg=PA129.
- ↑ Seel, Fritz; Ballreich, Kurt; Schmutzler, Reinhard (January 1962). "Darstellung von Säurefluoriden der Thiophosphonsäuren mittels Kaliumfluorsulfinats". Chemische Berichte 95 (1): 199–202. doi:10.1002/cber.19620950132.
- ↑ Doty, Leon F.; Cavell, Ronald G. (1 November 1974). "Mixed-valence derivatives of phosphorus sulfides. Two new isomeric thiophosphoryl-μ-thio-phosphines containing fluorine and trifluoromethyl substituents and a discussion of their exchange properties". Inorganic Chemistry 13 (11): 2722–2729. doi:10.1021/ic50141a035.
- ↑ .Ebsworth, E. A. V.; Macdonald, E. K.; Rankin, D. W. H. (1980). "The preparation, properties and gas-phase molecular structure of difluoro(germylthio)phosphine". Monatshefte für Chemie 111 (1): 221–233. doi:10.1007/BF00938730.
- ↑ Shimanouchi, T. (1973-04-01). "Tables of Molecular Vibrational Frequencies: Part 7". Journal of Physical and Chemical Reference Data 2 (2): 241. doi:10.1063/1.3253118. ISSN 0047-2689. Bibcode: 1973JPCRD...2..225S.
- ↑ Durig, J. R.; Clark, J. W. (1967-04-15). "Vibrational Spectra of SPCl3, SPCl2F, SPClF2, and SPF3". The Journal of Chemical Physics 46 (8): 3057–3068. doi:10.1063/1.1841177. ISSN 0021-9606. Bibcode: 1967JChPh..46.3057D.
- ↑ Horn, Hans-Georg; Müller, Achim (September 1966). "Untersuchungen an Phosphoryl- und Thiophosphorylverbindungen. IV.19F- und31P-kernmagnetische Resonanzspektren von SPF3, SPF2Cl und SPF2Br" (in de). Zeitschrift für anorganische und allgemeine Chemie 346 (5–6): 266–271. doi:10.1002/zaac.19663460506.
 |

