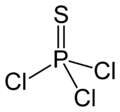
Chemistry:Thiophosphoryl chloride
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphorothioic trichloride
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1837 | ||
| |||
| |||
| Properties | |||
| PSCl 3 | |||
| Molar mass | 169.38 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.67 g/cm3 | ||
| Melting point | −35 °C (−31 °F; 238 K) | ||
| Boiling point | 125 °C (257 °F; 398 K) | ||
| Reacts | |||
| Solubility | Soluble in benzene, chloroform, CS 2 and CCl 4. | ||
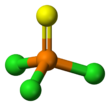
| Structure | |||
| Tetrahedral at the P atom | |||
| Hazards | |||
| Main hazards | Violent hydrolysis; releasing HCl on contact with water,[2] maybe corrosive to metals and skin | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H302, H314, H330 | |||
| P260, P264, P270, P271, P280, P284, P301+317Script error: No such module "Preview warning".Category:GHS errors, P301+330+331, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P320, P321, P330, P363, P403+233, P405, P501 | |||
| Flash point | none[3] | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Thiophosphoryl chloride is an inorganic compound with the chemical formula PSCl
3.[5] It is a colorless pungent smelling liquid that fumes in air. It is synthesized from phosphorus chloride and used to thiophosphorylate organic compounds, such as to produce insecticides.
Synthesis
Thiophosphoryl chloride can be generated by several reactions starting from phosphorus trichloride. The most common and practical synthesis, hence used in industrial manufacturing, is directly reacting phosphorus trichloride with excess sulfur at 180 °C.[6]
- PCl
3 + S → PSCl
3
Using this method, yields can be very high after purification by distillation. Catalysts facilitate the reaction at lower temperatures, but are not usually necessary. Alternatively, it is obtained by combining phosphorus pentasulfide and phosphorus pentachloride.[7]
- 3 PCl
5 + P
2S
5 → 5 PSCl
3
Structure
Thiophosphoryl chloride has tetrahedral molecular geometry and C3v molecular symmetry, with the structure S=PCl
3. According to gas electron diffraction, the phosphorus–sulfur bond length is 189 pm and the phosphorus–chlorine bond length is 201 pm, while the Cl–P–Cl bond angle is 102°.[8]
Reactions
PSCl
3 is soluble in benzene, carbon tetrachloride, chloroform, and carbon disulfide.[5] However, it hydrolyzes rapidly in basic or hydroxylic solutions, such as alcohols and amines, to produce thiophosphates.[6] In water PSCl
3 reacts, and contingent on the reaction conditions, produces either phosphoric acid, hydrogen sulfide, and hydrochloric acid or dichlorothiophosphoric acid and hydrochloric acid.[9]
- PSCl
3 + 4 H
2O → H
3PO
4 + H
2S + 3 HCl - PSCl
3 + H
2O → HO–P(=S)Cl
2 + HCl
PSCl
3 is used to thiophosphorylate organic compounds (to add thiophosphoryl group, P=S, with three free valences at the P atom, to organic compounds).[6] This conversion is widely applicable for amines and alcohols, as well as aminoalcohols, diols, and diamines.[5] Industrially, PSCl
3 is used to produce insecticides, like parathion.[9]
- PSCl
3 + 2 CH
3CH
2OH → (CH
3CH
2–O–)
2P(=S)–Cl + 2 HCl - (CH
3CH
2–O–)
2P(=S)–Cl + Na+
[−
O–C
6H
4–NO
2] → (CH
3CH
2–O–)
2P(=S)–O–C
6H
4–NO
2 + NaCl
PSCl
3 reacts with tertiary amides to generate thioamides.[5] For example:
- C
6H
5–C(=O)–N(–CH
3)
2 + PSCl
3 → C
6H
5–C(=S)–N(–CH
3)
2 + POCl
3
When treated with methylmagnesium iodide, it give tetramethyldiphosphine disulfide (H
3C–)
2P(=S)–P(=S)(–CH
3)
2.[10]
References
- ↑ Thiophosphoryl chloride: trade names
- ↑ Thiophosphoryl chloride: main hazards
- ↑ Thiophosphoryl chloride: flash point
- ↑ "Thiophosphoryl chloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/19883#section=Safety-and-Hazards.
- ↑ 5.0 5.1 5.2 5.3 Spilling, C. D. "Thiophosphoryl Chloride" in Encyclopedia of Reagents for Organic Synthesis John Wiley & Sons, Weinheim, 2001. doi: 10.1002/047084289X.rt104. Article Online Posting Date: April 15, 2001.
- ↑ 6.0 6.1 6.2 "Phosphorus Compounds, Inorganic". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a19_527. ISBN 3527306730.
- ↑ Martin, D. R.; Duvall, W. M. “Phosphorus(V) Sulfochloride” Inorganic Syntheses, 1953, Volume IV, p73. doi: 10.1002/9780470132357.ch24.
- ↑ Kuchitsu, Kozo; Moritani, Tohei; Morino, Yonezo (1971). "Molecular structures of phosphoryl fluoride, phosphoryl chloride, and thiophosphoryl chloride studied by gas electron diffraction". Inorg. Chem. 10 (2): 344–350. doi:10.1021/ic50096a025.
- ↑ 9.0 9.1 Fee, D. C.; Gard, D. R.; Yang, C. “Phosphorus Compounds” Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons: New York, 2005. doi: 10.1002/0471238961.16081519060505.a01.pub2
- ↑ G. W. Parshall "Tetramethylbiphosphine Disulfide" Org. Synth. 1965, volume 45, p. 102. doi:10.15227/orgsyn.045.0102
 |