Chemistry:Avasimibe
 | |
| Clinical data | |
|---|---|
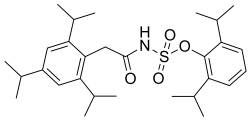
| Other names | 2,6-diisopropylphenyl (2-(2,4,6-triisopropylphenyl)acetyl)sulfamate |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP3A4, 2C9) |
| Elimination half-life | 15–24 hours |
| Excretion | Fecal (predominant), renal (<2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C29H43NO4S |
| Molar mass | 501.73 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Avasimibe (INN), codenamed CI 1011, is a drug that inhibits sterol O-acyltransferases (SOAT1 and SOAT2, also known as ACAT1 and ACAT2), enzymes involved in the metabolism and catabolism of cholesterol. It was discovered by Parke-Davis (later Pfizer) and developed as a possible lipid-lowering agent and treatment for atherosclerosis.[1]
The first description of avasimibe was published in 1996.[2] Clinical trials began in 1997.[1] However, development was halted in 2003 due to a high potential for interactions with other medicines,[3] and a pivotal study found it had no favorable effect on atherosclerosis and actually increased LDL cholesterol levels significantly.[4]
SOAT/ACAT inhibition has since been discredited as a viable strategy for treating high cholesterol and atherosclerosis,[5] but renewed interest in avasimibe has arisen due to its potential antitumor utility through other mechanisms.
It has never been marketed or used outside clinical trials.[6]
Pharmacology
Mechanism of action
Avasimibe is a potent activator of the pregnane X receptor and, consequently, an indirect inducer of CYP3A4 and P-glycoprotein, as well as a potent inhibitor of several cytochrome P450 isoenzymes, including CYP1A2, CYP2C9, and CYP2C19; its spectrum of CYP induction and inhibition is similar to that of rifampicin.[7][8]
Pharmacokinetics
Avasimibe is better absorbed when taken with food, especially with a high-fat meal, as reflected by increases in its peak serum concentration and AUC.[1]
History
Avasimibe was the result of a rational drug design process carried out at Parke-Davis in the early 1990s which sought to obtain orally bioavailable, water-soluble ACAT inhibitors; all such inhibitors known at the time were lipophilic and poorly absorbed when taken by mouth.[9] This process yielded several compounds with potential, including one (designated PD 138142-15) with good solubility in water and remarkable efficacy in animal studies, but it was chemically unstable and degraded rapidly, especially in acidic environments.[2] (Undesirable CYP450 induction was first noted at this time, in PD 138142-15 and its degradation products.[2][10]) Chemical modification of PD 138142-15 and [[retrosynthetic analysis found that avasimibe (then codenamed CI-1011) could be easily manufactured from commercially available starting compounds, and once its efficacy was demonstrated in vitro and in rat studies, it was selected for further development.[2]
After additional safety and preclinical efficacy studies in animals, phase I clinical trials in humans began in 1997, first for hyperlipidemia (June) and subsequently for atherosclerosis (December).[1][6] Phase II trials for both indications followed in 1998, and phase III trials in 2001.[1][6]
In October 2003, clinical development of avasimibe was discontinued.[6] Later research discredited the concept of ACAT inhibition as a treatment for dyslipidemia and atherosclerosis, and interest in these compounds as a class waned accordingly.[11][5]
Research
Since the termination of its development as an antilipidemic agent, there has been renewed interest in potential repurposing of avasimibe as an antitumor drug[12][13] and to prevent or treat bacterial infections by decreasing bacterial virulence.[14] (As of 2022), these potential indications remain in preclinical research.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Avasimibe. CI 1011". Drugs in R&D 3 (3): 173–4. 2002. doi:10.2165/00126839-200203030-00005. PMID 12099161.
- ↑ 2.0 2.1 2.2 2.3 "Inhibitors of acyl-CoA: cholesterol O-acyl transferase (ACAT) as hypocholesterolemic agents. CI-1011: an acyl sulfamate with unique cholesterol-lowering activity in animals fed noncholesterol-supplemented diets". J Med Chem 39 (26): 5031–4. December 1996. doi:10.1021/jm960674d. PMID 8978833.
- ↑ "Avasimibe (Code C75252)". https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=ncit&code=C75252.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions". Circulation 110 (21): 3372–7. November 2004. doi:10.1161/01.CIR.0000147777.12010.EF. PMID 15533865.
- ↑ 5.0 5.1 "The treatment of dyslipidemia--what's left in the pipeline?". ChemMedChem 3 (2): 206–21. February 2008. doi:10.1002/cmdc.200700165. PMID 17963209.
- ↑ 6.0 6.1 6.2 6.3 "Drug Profile: Avasimibe" (PDF). AdisInsight. 2003-10-28. https://adisinsight.springer.com/drugs/800008778. Retrieved 2022-05-09.
- ↑ "Avasimibe induces CYP3A4 and multiple drug resistance protein 1 gene expression through activation of the pregnane X receptor". J Pharmacol Exp Ther 306 (3): 1027–34. September 2003. doi:10.1124/jpet.103.050526. PMID 12766253.
- ↑ "Effects of avasimibe on cytochrome P450 2C9 expression in vitro and in vivo". Drug Metab Dispos 32 (12): 1370–6. December 2004. doi:10.1124/dmd.104.000208. PMID 15333513.
- ↑ "Inhibitors of acyl-CoA: cholesterol O-acyl transferase (ACAT) as hypocholesterolemic agents. 6. The first water-soluble ACAT inhibitor with lipid-regulating activity". J Med Chem 37 (5): 560–2. March 1994. doi:10.1021/jm00031a002. PMID 8126693.
- ↑ Hepatic microsomal induction profile of carbamic acid <nowiki>journal=Biochem Pharmacol. 49. March 1995. pp. 799–808. doi:10.1016/0006-2952(94)00540-3. PMID 7702638.
- ↑ "Effect of ACAT inhibition on the progression of coronary atherosclerosis". N Engl J Med 354 (12): 1253–63. March 2006. doi:10.1056/NEJMoa054699. PMID 16554527.
- ↑ "Pharmacologic and genetic inhibition of cholesterol esterification enzymes reduces tumour burden: A systematic review and meta-analysis of preclinical models". Biochem Pharmacol 196: 114731. February 2022. doi:10.1016/j.bcp.2021.114731. PMID 34407453. https://eprints.whiterose.ac.uk/177472/7/CRediT.pdf. Retrieved 15 August 2022.
- ↑ "Acyl-Coenzyme A: Cholesterol Acyltransferase Inhibition in Cancer Treatment". Anticancer Res 39 (7): 3385–3394. July 2019. doi:10.21873/anticanres.13482. PMID 31262860.
- ↑ "Repurposing Avasimibe to Inhibit Bacterial Glycosyltransferases". Pathogens 11 (3): 370. March 2022. doi:10.3390/pathogens11030370. PMID 35335693.
 |

