Chemistry:Methaneseleninic acid
| |
| Names | |
|---|---|
| IUPAC name
Methaneseleninic acid
| |
| Other names
Methylseleninic acid
MSA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
| MeSH | C008493 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
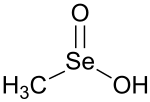
| CH 3SeO 2H | |
| Molar mass | 127.012 g·mol−1 |
| Appearance | White crystalline solid or powder[1] |
| Odor | Stench[1] |
| Melting point | 128–132 °C (262–270 °F; 401–405 K)[1] |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H301, H314, H331, H373, H410 | |
| P260, P261, P264, P270, P271, P273, P280, P301+310, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P311, P314, P321, P330, P363, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methaneseleninic acid (methylseleninic acid or MSA) is an organoselenium compound, a seleninic acid with the chemical formula CH
3SeO
2H. Its structure is CH
3–Se(=O)–OH.
Preparation
Methaneseleninic acid is conveniently synthesized through oxidation of commercially available dimethyl diselenide by 3% hydrogen peroxide.[2]
- CH
3–Se–Se–CH
3 + H
2O
2 → 2 CH
3–Se(=O)–OH
Seleninic acids can be prepared by oxidation of selenoesters with one equivalent of dimethyldioxirane (DMDO). Use of excess DMDO affords little studied selenonic acids (R–Se(=O)
2–OH).[3]
- R–Se–C(=O)–R' + DMDO → R–Se(=O)–OH
- R–Se–C(=O)–R' + excess DMDO → R–Se(=O)
2–OH
Selenenic acids, formed during the syn-elimination of selenoxides, undergo spontaneous disproportionation into the corresponding seleninic acids and diselenides:
- 4 R–Se–OH → 2 R–Se(=O)–OH + R–Se–Se–R
Structure, bonding, properties
Methaneseleninic acid, from decomposition of Se-methylselenocysteine Se-oxide but also available commercially, has been characterized by X-ray crystallography.[4] The configuration about the selenium atom is pyramidal, with Se-C = 1.925(8) Å, Se-O = 1.672(7) Å, Se-OH = 1.756(7) Å, the angle OSeO = 103.0(3)°, the angle HO-Se-C = 93.5(3)°, and the angle OSeC = 101.4(3)°. The structure is isomorphous to that of methanesulfinic acid [5] Optical isomers of methaneseleninic acid can be isolated as chiral crystals by recrystallization from a mixture of methanol and toluene. The absolute configuration of one of the enantiomers was determined by X-ray crystallography. Optically active methaneseleninic acid was stable toward racemization in the solid state, although it racemized very rapidly in solution.[6]
Anticancer activity
Methaneseleninic acid shows potential anticancer activity and is a model for studying the anticancer effects of selenium in vitro.[7] Methaneseleninic acid shows superior in vivo inhibitory efficacy toward human prostate cancer compared to selenomethionine or selenite (ion).[8] It has recently been reported that methaneseleninic acid enhances the efficacy of paclitaxel for treatment of triple-negative breast cancer,[9] that methaneseleninic acid functions as an aromatase inhibitor, of possible use in therapy for estrogen receptor-positive breast cancer in postmenopausal women,[10] that methaneseleninic acid shows promise as a sensitizing agent for apoptosis induced by the Bcl-2-family inhibitor ABT-737 in several cancer lines,[11] and that methaneseleninic acid restricts tumor growth in the nude mouse model of metastatic breast cancer[12] and Lewis lung carcinoma in mice.[13]
Methaneselenol (CH
3SeH) can be produced in vivo by reduction of methaneseleninic acid and may in fact be the key metabolite responsible for selenium’s anticancer activity[2][14] through generation of superoxide.[15] The reduction of methaneseleninic acid by mammalian thioredoxin reductase has been studied.[16]
References
- ↑ 1.0 1.1 1.2 https://www.sigmaaldrich.com/US/en/sds/aldrich/541281?userType=anonymous
- ↑ 2.0 2.1 Ip, C.; Thompson, H.J.; Zhu, Z.; Ganther, H.E. (2000). "In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention". Cancer Res. 60 (11): 2882–2886. PMID 10850432.
- ↑ Abdo, M.; Knapp, S. (2008). "Biomimetic seleninates and selenonates". J. Am. Chem. Soc. 130 (29): 9234–9235. doi:10.1021/ja8034448. PMID 18576651.
- ↑ Block, E.; Birringer, M.; Jiang, W.; Nakahodo, T.; Thompson, H. J.; Toscano, P. J.; Uzar, H.; Zhang, X. et al. (2001). "Allium chemistry: Synthesis, natural occurrence, biological activity, and chemistry of Se-alk(en)ylselenocysteines and their γ-glutamyl derivatives and oxidation products". J. Agric. Food Chem. 49 (1): 458–470. doi:10.1021/jf001097b. PMID 11305255.
- ↑ Seff, K.; Heidner, E. G.; Meyers, M.; Trueblood, K. N. (1969). "The crystal and molecular structure of methanesulfinic acid". Acta Crystallographica Section B 25 (2): 350–354. doi:10.1107/s0567740869002214.
- ↑ Nakashima, Y.; Shimizu, T.; Hirabayashi, K.; Yasui, M.; Nakazato, M.; Iwasaki, F.; Kamigata, N. (2005). "Optically active seleninic acid: Isolation, absolute configuration, stability, and chiral crystallization". Bull. Chem. Soc. Jpn. 78 (4): 710–714. doi:10.1246/bcsj.78.710.
- ↑ Zhao, H.; Whitfield, M. L.; Xu, T.; Botstein, D.; Brooks, J. D. (2003). "Diverse effects of methylseleninic acid on the transcriptional program of human prostate cancer cells". Molecular Biology of the Cell 15 (2): 506–519. doi:10.1091/mbc.E03-07-0501. PMID 14617803.
- ↑ Li, G. X.; Lee, H. J.; Wang, Z.; Hu, H.; Liao, J. D.; Watts, J. C.; Combs, G. F. Jr.; Lu, J. (2008). "Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite". Carcinogenesis 29 (5): 1005–1012. doi:10.1093/carcin/bgn007. PMID 18310093.
- ↑ Qi, Y.; Fu, X.; Xiong, Z.; Zhang, H.; Hill, S.M.; Rowan, B.G.; Dong, Y. (2012). "Methylseleninic acid enhances paclitaxel efficacy for the treatment of triple-negative breast cancer". PLOS ONE 7 (2): e31539. doi:10.1371/journal.pone.0031539. PMID 22348099. Bibcode: 2012PLoSO...731539Q.
- ↑ Gao, R.; Zhao, L.; Liu, X.; Rowan, B.G.; Wabitsch, M.; Edwards, D.P.; Nishi, Y.; Yanase, T. et al. (2012). "Methylseleninic acid is a novel suppressor of aromatase expression". J. Endocrinol. 212 (2): 199–205. doi:10.1530/JOE-11-0363. PMID 22128327.
- ↑ Yin, S; Dong, Y; Li, J; Fan, L; Wang, L; Lu, J; Vang, O; Hu, H (2012). "Methylseleninic acid potentiates multiple types of cancer cells to ABT-737-induced apoptosis by targeting Mcl-1 and Bad". Apoptosis 17 (4): 388–399. doi:10.1007/s10495-011-0687-9. PMID 22179721.
- ↑ Wu, X.; Zhang, Y.; Pei, Z.; Chen, S.; Yang, X.; Chen, Y.; Lin, D.; Ma, R.Z. (2012). "Methylseleninic acid restricts tumor growth in nude mice model of metastatic breast cancer probably via inhibiting angiopoietin-2". BMC Cancer 12: 192. doi:10.1186/1471-2407-12-192. PMID 22640261.
- ↑ Yan, L.; DeMars, L.C. (2012). "Dietary supplementation with methylseleninic acid, but not selenomethionine, reduces spontaneous metastasis of Lewis lung carcinoma in mice". Int. J. Cancer 131 (6): 1260–1266. doi:10.1002/ijc.27355. PMID 22095442.
- ↑ Ip, C.; Dong, Y.; Ganther, H. E. (2002). "New concepts in selenium chemoprevention". Cancer Metastasis Rev. 21 (3–4): 281–289. doi:10.1023/A:1021263027659. PMID 12549766.
- ↑ Spallholz, J.E.; Shriver, B.J.; Reid, T.W. (2001). "Dimethyldiselenide and methylseleninic acid generate superoxide in an In vitro chemiluminescence assay in the presence of glutathione: Implications for the anticarcinogenic activity of L-selenomethionine and L-Se-methylselenocysteine". Nutrition and Cancer 40 (1): 34–41. doi:10.1207/S15327914NC401_8. PMID 11799920.
- ↑ Snider, G.; Grout, L.; Ruggles, E. L.; Hondal, R. J. (2010). "Methaneseleninic acid is a substrate for truncated mammalian thioredoxin reductase: Implications for the catalytic mechanism and redox signaling". Biochemistry 49 (48): 10329–10338. doi:10.1021/bi101130t. PMID 21038895.
 |

