Biology:Thioredoxin reductase
| Thioredoxin reductase | |
|---|---|
| Identifiers | |
| Symbol | ? |
| InterPro | IPR005982 |
| PROSITE | PS00573 |
| SCOP2 | 1zof / SCOPe / SUPFAM |
Thioredoxin reductases (TR, TrxR) (EC 1.8.1.9) are enzymes that reduce thioredoxin (Trx).[1] Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. In bacteria TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH.[2][3][4] Both classes are flavoproteins which function as homodimers. Each monomer contains a FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond.[5]
Cellular role
Thioredoxin reductases are enzymes that catalyze the reduction of thioredoxin[1] and hence they are a central component in the thioredoxin system. Together with thioredoxin (Trx) and NADPH this system's most general description is as a system for reducing disulfide bonds in cells. Electrons are taken from NADPH via TrxR and are transferred to the active site of Trx, which goes on to reduce protein disulfides or other substrates.[6] The Trx system exists in all living cells and has an evolutionary history tied to DNA as a genetic material, defense against oxidative damage due to oxygen metabolism, and redox signaling using molecules like hydrogen peroxide and nitric oxide.[7][8]

Diversity
Two classes of thioredoxin reductase have evolved independently:
- A high molecular weight (MW = ~55,000) type containing a selenocysteine residue in its active site has been identified in higher eukaryotes including humans. This TxR is related to glutathione reductase, trypanothione reductase, mercuric reductase and lipoamide dehydrogenase.[5]
- A low molecular weight (MW = ~ 35,000) type has been identified in archaea, bacteria and other eukarya.[5]
These two classes of TrxR have only ~20% sequence identity in the section of primary sequence where they can be reliably aligned.[5] The net reaction of both classes of TrxR is identical but the mechanism of action of each is distinct.[9]
Humans express three thioredoxin reductase isozymes: thioredoxin reductase 1 (TrxR1, cytosolic), thioredoxin reductase 2 (TrxR2, mitochondrial), thioredoxin reductase 3 (TrxR3, testis specific).[10] Each isozyme is encoded by a separate gene:
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Structure
E. coli
In E. coli ThxR there are two binding domains, one for FAD and another for NADPH. The connection between these two domains is a two-stranded anti-parallel β-sheet.[11] Each domain individually is very similar to the analogous domains in glutathione reductase, and lipoamide dehydrogenase but they relative orientation of these domains in ThxR is rotated by 66 degrees.[11] This becomes significant in the enzyme mechanism of action which is described below. ThxR homo-dimerizes with the interface between the two monomers formed by three alpha-helices and two loops.[11] Each monomer can separately bind a molecule of thioredoxin.
Mammalian
Mammalian TrxR structure is similar to E. coli. It contains a FAD and NADPH binding domain, and an interface between two monomer subunits. In mammalian ThxR there is an insertion in the FAD binding domain between two alpha helices which forms a small pair of beta strands.[12] The active disulfide in the enzyme is located on one of these helices and thus the active disulfide bond is located in the FAD domain and not the NADPH domain as in E. coli and other prokaryotes.[12]
Mechanism
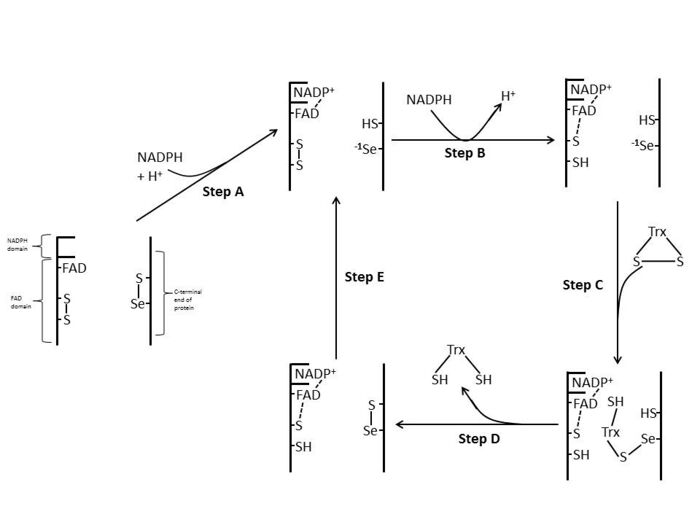
E. coli
In E. coli ThxR the spatial orientation of the FAD and NADPH domains are such that the redox-active rings of FAD and NADPH are not in close proximity to each other.[1] When the FAD domain of E. coli is rotated 66 degrees with the NADPH domain remaining fixed the two prosthetic groups move into close contact allowing electrons to pass from NADPH to FAD and then to the active site disulfide bond.[1][15] The conserved active site residues in E. coli are -Cys-Ala-Thr-Cys-.[1]
Mammalian
Mammalian TrxRs have a much higher sequence homology with glutathione reductase than E. coli.[1] The active-site Cys residues in the FAD domain and bound NADPH domain are in close proximity removing the necessity for a 66 degree rotation for electron transfer found in E. coli. An additional feature of the mammalian mechanism is the presence of a selenocysteine residue at the C-terminal end of the protein which is required for catalytic activity. The conserved residues in mammalian active site are -Cys-Val-Asn-Val-Gly-Cys-.[1]
Detection methods
Thioredoxin reductase can be quantified by various methods such as the DTNB assay using Ellman's reagent. The disulfide-based TRFS series of fluorescent probes have shown selective detection of TrxR.[16][17][18][19] Mafireyi synthesized the first diselenide probe that was applied in the detection of TrxR.[20][21] Other detection methods include immunological techniques and the selenocystine-thioredoxin reductase assay (SC-TR assay).
Clinical significance
Cancer treatment
Since the activity of this enzyme is essential for cell growth and survival, it is a good target for anti-tumor therapy. Furthermore, the enzyme is upregulated in several types of cancer, including malignant mesothelioma.[22][23] For example, motexafin gadolinium (MGd) is a new chemotherapeutic agent that selectively targets tumor cells, leading to cell death and apoptosis via inhibition of thioredoxin reductase and ribonucleotide reductase.
Cardiomyopathy
Dilated cardiomyopathy (DCM) is a common diagnosis in cases of congestive heart failure. Thioredoxin reductases are essential proteins for regulating cellular redox balance and mitigating the damage caused by reactive oxygen species generated via oxidative phosphorylation in the mitochondria. Inactivation of mitochondrial TrxR2 in mice results in thinning of the ventricular heart walls and neonatal death.[10] Furthermore two mutations in the TrxR2 gene are found in patients diagnosed with DCM and not in a control population. It is hypothesized that the pathological impact of these mutations is an impaired ability to control oxidative damage in cardiac myocytes.[24]
Antibiotic
There has recently been some research to show that low molecular weight thioredoxin reductase could be a target for novel antibiotics (such as auranofin or Ebselen.[25]) This is especially true for Mycobacterium Haemophilum, and could be used for antibiotic resistant bacteria.[26]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Thioredoxin reductase". The Biochemical Journal 346 Pt 1 (1): 1–8. February 2000. doi:10.1042/0264-6021:3460001. PMID 10657232.
- ↑ "Characterization of Escherichia coli NrdH. A glutaredoxin-like protein with a thioredoxin-like activity profile". The Journal of Biological Chemistry 272 (29): 18044–50. July 1997. doi:10.1074/jbc.272.29.18044. PMID 9218434.
- ↑ "The crystal structure of Mycobacterium tuberculosis NrdH at 0.87 Å suggests a possible mode of its activity". Biochemistry 52 (23): 4056–65. June 2013. doi:10.1021/bi400191z. PMID 23675692.
- ↑ "Redox Proteins of Mycobacterium tuberculosis" (in en). Journal of the Indian Institute of Science 94 (1): 127–138. 2014-01-01. ISSN 0970-4140. http://journal.library.iisc.ernet.in/index.php/iisc/article/view/4461.
- ↑ 5.0 5.1 5.2 5.3 "The diversity and evolution of thioredoxin reductase: new perspectives". Trends in Parasitology 18 (7): 302–8. July 2002. doi:10.1016/S1471-4922(02)02293-6. PMID 12379950.
- ↑ 6.0 6.1 "Thioredoxin and thioredoxin reductase: current research with special reference to human disease". Biochemical and Biophysical Research Communications 396 (1): 120–4. May 2010. doi:10.1016/j.bbrc.2010.03.083. PMID 20494123. https://zenodo.org/record/1065567.
- ↑ "Thioredoxins and glutaredoxins: unifying elements in redox biology". Annual Review of Genetics 43: 335–67. 2009. doi:10.1146/annurev-genet-102108-134201. PMID 19691428.
- ↑ "Thioredoxin and related molecules--from biology to health and disease". Antioxidants & Redox Signaling 9 (1): 25–47. Jan 2007. doi:10.1089/ars.2007.9.25. PMID 17115886.
- ↑ "The mechanism of thioredoxin reductase from human placenta is similar to the mechanisms of lipoamide dehydrogenase and glutathione reductase and is distinct from the mechanism of thioredoxin reductase from Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America 94 (8): 3621–6. Apr 1997. doi:10.1073/pnas.94.8.3621. PMID 9108027. Bibcode: 1997PNAS...94.3621A.
- ↑ 10.0 10.1 "Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function". Molecular and Cellular Biology 24 (21): 9414–23. Nov 2004. doi:10.1128/MCB.24.21.9414-9423.2004. PMID 15485910.
- ↑ 11.0 11.1 11.2 "Mechanism and structure of thioredoxin reductase from Escherichia coli". FASEB Journal 9 (13): 1267–76. Oct 1995. doi:10.1096/fasebj.9.13.7557016. PMID 7557016.
- ↑ 12.0 12.1 "Three-dimensional structure of a mammalian thioredoxin reductase: implications for mechanism and evolution of a selenocysteine-dependent enzyme". Proceedings of the National Academy of Sciences of the United States of America 98 (17): 9533–8. Aug 2001. doi:10.1073/pnas.171178698. PMID 11481439. Bibcode: 2001PNAS...98.9533S.
- ↑ "Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence". Proceedings of the National Academy of Sciences of the United States of America 97 (11): 5854–9. May 2000. doi:10.1073/pnas.100114897. PMID 10801974. Bibcode: 2000PNAS...97.5854Z.
- ↑ "Human thioredoxin reductase is efficiently inhibited by (2,2':6',2' '-terpyridine)platinum(II) complexes. Possible implications for a novel antitumor strategy". Journal of Medicinal Chemistry 44 (17): 2784–92. Aug 2001. doi:10.1021/jm001014i. PMID 11495589.
- ↑ "Reductive half-reaction of thioredoxin reductase from Escherichia coli". Biochemistry 36 (31): 9464–77. Aug 1997. doi:10.1021/bi970307j. PMID 9235991.
- ↑ "A fast and specific fluorescent probe for thioredoxin reductase that works via disulphide bond cleavage". Nature Communications 10 (1): 2745. June 2019. doi:10.1038/s41467-019-10807-8. PMID 31227705. Bibcode: 2019NatCo..10.2745L.
- ↑ "A fast response and red emission probe for mammalian thioredoxin reductase". Chemical Communications 52 (81): 12060–12063. October 2016. doi:10.1039/C6CC04984B. PMID 27709154.
- ↑ "Loss of thioredoxin reductase function in a mouse stroke model disclosed by a two-photon fluorescent probe". Chemical Communications 56 (90): 14075–14078. November 2020. doi:10.1039/D0CC05900E. PMID 33107534.
- ↑ "A small molecule probe reveals declined mitochondrial thioredoxin reductase activity in a Parkinson's disease model". Chemical Communications 52 (11): 2296–9. February 2016. doi:10.1039/c5cc09998f. PMID 26725656.
- ↑ "A Diselenide Turn-On Fluorescent Probe for the Detection of Thioredoxin Reductase". Angewandte Chemie 59 (35): 15147–15151. August 2020. doi:10.1002/ange.202004094. PMID 32449244.
- ↑ "Fluorogenic probes for thioredoxin reductase activity" (in en). Results in Chemistry 3: 100127. 2021-03-29. doi:10.1016/j.rechem.2021.100127. ISSN 2211-7156.
- ↑ "Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress". Free Radical Biology & Medicine 41 (6): 874–85. Sep 2006. doi:10.1016/j.freeradbiomed.2006.04.031. PMID 16934670.
- ↑ "Up-regulation of thioredoxin and thioredoxin reductase in human malignant pleural mesothelioma". International Journal of Cancer 95 (3): 198–204. May 2001. doi:10.1002/1097-0215(20010520)95:3<198::AID-IJC1034>3.0.CO;2-F. PMID 11307155.
- ↑ "Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy". European Heart Journal 32 (9): 1121–33. May 2011. doi:10.1093/eurheartj/ehq507. PMID 21247928.
- ↑ "Aspergillus fumigatus Thioredoxin Reductase". Antimicrobial Agents and Chemotherapy 63 (3). March 2019. doi:10.1128/AAC.02281-18. PMID 30642940.
- ↑ "Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis". Proceedings of the National Academy of Sciences of the United States of America 112 (14): 4453–8. April 2015. doi:10.1073/pnas.1504022112. PMID 25831516. Bibcode: 2015PNAS..112.4453H.
External links
- Thioredoxin+Reductase+(NADPH) at the US National Library of Medicine Medical Subject Headings (MeSH)
 |




