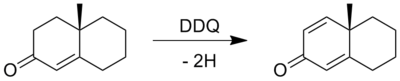Chemistry:Dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At its simplest, it's a useful way of converting alkanes, which are relatively inert and thus low-valued, to olefins, which are reactive and thus more valuable. Alkenes are precursors to aldehydes (R–CH=O), alcohols (R–OH), polymers, and aromatics.[1] As a problematic reaction, the fouling and inactivation of many catalysts arises via coking, which is the dehydrogenative polymerization of organic substrates.[2]
Enzymes that catalyze dehydrogenation are called dehydrogenases.
Heterogeneous catalytic routes
Styrene
Dehydrogenation processes are used extensively to produce aromatics in the petrochemical industry. Such processes are highly endothermic and require temperatures of 500 °C and above.[1][3] Dehydrogenation also converts saturated fats to unsaturated fats. One of the largest scale dehydrogenation reactions is the production of styrene by dehydrogenation of ethylbenzene. Typical dehydrogenation catalysts are based on iron(III) oxide, promoted by several percent potassium oxide or potassium carbonate.[4]
Other alkenes
The cracking processes especially fluid catalytic cracking and steam cracker produce high-purity mono-olefins from paraffins. Typical operating conditions use chromium (III) oxide catalyst at 500 °C. Target products are [propylene]], butenes, and isopentane, etc. These simple compounds are important raw materials for the synthesis of polymers and gasoline additives.
Oxidative dehydrogenation
Relative to thermal cracking of alkanes, oxidative dehydrogenation (ODH) is of interest for two reasons: (1) undesired reactions take place at high temperature leading to coking and catalyst deactivation, making frequent regeneration of the catalyst unavoidable, (2) thermal dehydrogenation is expensive as it requires a large amount of heat. Oxidative dehydrogenation (ODH) of n-butane is an alternative to classical dehydrogenation, steam cracking and fluid catalytic cracking processes.[5][6]
Formaldehyde is produced industrially by oxidative dehydrogenation of methanol. This reaction can also be viewed as a dehydrogenation using O
2 as the acceptor. The most common catalysts are silver metal, iron(III) oxide,[7] iron molybdenum oxides [e.g. iron(III) molybdate] with a molybdenum-enriched surface,[8] or vanadium oxides. In the commonly used formox process, methanol and oxygen react at ca. 250–400 °C in presence of iron oxide in combination with molybdenum and/or vanadium to produce formaldehyde according to the chemical equation:[9]
- CH
3OH + O
2 → 2 CH
2O + 2 H
2O
Homogeneous catalytic routes
A variety of dehydrogenation processes have been described for organic compounds. These dehydrogenation is of interest in the synthesis of fine organic chemicals.[10] Such reactions often rely on transition metal catalysts.[11][12] Dehydrogenation of unfunctionalized alkanes can be effected by homogeneous catalysis. Especially active for this reaction are pincer complexes.[13][14]
Stoichiometric processes
Dehydrogenation of amines to nitriles using a variety of reagents, such as Iodine pentafluoride (IF5).
In typical aromatization, six-membered alicyclic rings, e.g. cyclohexene, can be aromatized in the presence of hydrogenation acceptors. The elements sulfur and selenium promote this process. On the laboratory scale, quinones, especially 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) are effective.
Main group hydrides

The dehydrogenative coupling of silanes has also been developed.[15]
- [math]\ce{ \mathit{n}\ PhSiH3 -> {}[PhSiH]_\mathit{n}{} + \mathit{n}\ H2 }[/math]
The dehydrogenation of amine-boranes is related reaction. This process once gained interests for its potential for hydrogen storage.[16]
References
- ↑ 1.0 1.1 Wittcoff, Harold A.; Reuben, Bryan G.; Plotkin, Jeffrey S. (2004). Industrial Organic Chemicals, Second Edition - Wittcoff - Wiley Online Library. doi:10.1002/0471651540. ISBN 9780471651543.
- ↑ Guisnet, M.; Magnoux, P. (2001). "Organic chemistry of coke formation". Applied Catalysis A: General 212 (1–2): 83–96. doi:10.1016/S0926-860X(00)00845-0.
- ↑ Survey of Industrial Chemistry | Philip J. Chenier | Springer. ISBN 9780471651543. https://www.springer.com/us/book/9780306472466.
- ↑ Denis H. James William M. Castor, “Styrene” in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- ↑ Ajayi, B. P.; Jermy, B. Rabindran; Ogunronbi, K. E.; Abussaud, B. A.; Al-Khattaf, S. (2013-04-15). "n-Butane dehydrogenation over mono and bimetallic MCM-41 catalysts under oxygen free atmosphere". Catalysis Today. Challenges in Nanoporous and Layered Materials for Catalysis 204: 189–196. doi:10.1016/j.cattod.2012.07.013.
- ↑ Polypropylene Production via Propane Dehydrogenation part 2, Technology Economics Program. by Intratec. 2012. ISBN 978-0615702162. http://www.slideshare.net/intratec/propylene-production-via-propane-dehydrogenation-part-2.
- ↑ Wang, Chien-Tsung; Ro, Shih-Hung (2005-05-10). "Nanocluster iron oxide-silica aerogel catalysts for methanol partial oxidation" (in en). Applied Catalysis A: General 285 (1): 196–204. doi:10.1016/j.apcata.2005.02.029. ISSN 0926-860X. https://www.sciencedirect.com/science/article/pii/S0926860X05001523.
- ↑ Dias, Ana Paula Soares; Montemor, Fátima; Portela, Manuel Farinha; Kiennemann, Alain (2015-02-01). "The role of the suprastoichiometric molybdenum during methanol to formaldehyde oxidation over Mo–Fe mixed oxides" (in en). Journal of Molecular Catalysis A: Chemical 397: 93–98. doi:10.1016/j.molcata.2014.10.022. ISSN 1381-1169. https://www.sciencedirect.com/science/article/pii/S1381116914004774.
- ↑ Günther Reuss, Walter Disteldorf, Armin Otto Gamer, Albrecht Hilt “Formaldehyde” in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_619
- ↑ Yeung, Charles S.; Dong, Vy M. (2011). "Catalytic Dehydrogenative Cross-Coupling: Forming Carbon−Carbon Bonds by Oxidizing Two Carbon−Hydrogen Bonds". Chemical Reviews 111 (3): 1215–1292. doi:10.1021/cr100280d. PMID 21391561.
- ↑ Dobereiner, Graham E.; Crabtree, Robert H. (2010). "Dehydrogenation as a Substrate-Activating Strategy in Homogeneous Transition-Metal Catalysis". Chemical Reviews 110 (2): 681–703. doi:10.1021/cr900202j. PMID 19938813.
- ↑ Choi, Jongwook; MacArthur, Amy H. Roy; Brookhart, Maurice; Goldman, Alan S. (2011). "Dehydrogenation and Related Reactions Catalyzed by Iridium Pincer Complexes". Chemical Reviews 111 (3): 1761–1779. doi:10.1021/cr1003503. PMID 21391566.
- ↑ "1". Alkane C-H Activation by Single-Site Metal Catalysis | Pedro J. Pérez | Springer. pp. 1–15. https://www.springer.com/us/book/9789048136971.
- ↑ Findlater, Michael; Choi, Jongwook; Goldman, Alan S.; Brookhart, Maurice (2012-01-01). Pérez, Pedro J.. ed (in en). Alkane C-H Activation by Single-Site Metal Catalysis. Catalysis by Metal Complexes. Springer Netherlands. pp. 113–141. doi:10.1007/978-90-481-3698-8_4. ISBN 9789048136971.
- ↑ Aitken, C.; Harrod, J. F.; Gill, U. S. (1987). "Structural studies of oligosilanes produced by catalytic dehydrogenative coupling of primary organosilanes". Can. J. Chem. 65 (8): 1804–1809. doi:10.1139/v87-303.
- ↑ Staubitz, Anne; Robertson, Alasdair P. M.; Manners, Ian (2010). "Ammonia-Borane and Related Compounds as Dihydrogen Sources". Chemical Reviews 110 (7): 4079–4124. doi:10.1021/cr100088b. PMID 20672860.
 |