Chemistry:Iodine pentafluoride
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Iodine(V) fluoride | |||
| Systematic IUPAC name
Pentafluoro-λ5-iodane | |||
| Other names
Iodic fluoride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| IF5 | |||
| Molar mass | 221.89 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 3.250 g/cm3 | ||
| Melting point | 9.43 °C (48.97 °F; 282.58 K) | ||
| Boiling point | 97.85 °C (208.13 °F; 371.00 K) | ||
| Reacts | |||
| −58.1·10−6 cm3/mol | |||
| Viscosity | 2.111 mPa·s | ||
| Structure | |||
| Monoclinic point group C2/c | |||
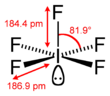
| Square pyramidal | |||
| square pyramidal[1] | |||
| Hazards | |||
| Main hazards | Toxic, oxidiser, corrosive, reacts with water to release HF | ||
| Safety data sheet | External MSDS | ||
| GHS pictograms |    
| ||
| GHS Signal word | Danger | ||
| H271, H301+311+331Script error: No such module "Preview warning".Category:GHS errors, H314, H371, H410[2] | |||
| P202, P232, P304, P310[2] | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Other anions
|
Iodine pentoxide | ||
Other cations
|
Bromine pentafluoride | ||
Related compounds
|
Iodine monofluoride Iodine trifluoride Iodine heptafluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses.[3]
Preparation
It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas.[4] This exothermic reaction is still used to produce iodine pentafluoride, although the reaction conditions have been improved.[5]
- I2 + 5 F2 → 2 IF5
Reactions
IF5 reacts vigorously with water forming hydrofluoric acid and iodic acid:
- IF5 + 3 H2O → HIO3 + 5 HF
Upon treatment with fluorine, it converts to iodine heptafluoride:[6]
- IF5 + F2 → IF7
It has been used as a solvent for handling metal fluorides. For example, the reduction of osmium hexafluoride to osmium pentafluoride with iodine is conducted in a solution in iodine pentafluoride:[7]
- 10 OsF6 + I2 → 10 OsF5 + 2 IF5
Primary amines react with iodine pentafluoride forming nitriles after hydrolysis.[8]
References
- ↑ Durbank, R. D.; Jones, G. R. (1974). "Crystal structure of Iodine Pentafluoride at -80°". Inorganic Chemistry 13 (5): 421–439. doi:10.1021/ic50135a012.
- ↑ 2.0 2.1 http://www.chemadvisor.com/Matheson/database/msds/mat11440000800003.PDF
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Moissan, M. H. (1891). "Nouvelles Recherches sur le Fluor". Annales de Chimie et de Physique 6 (24): 224–282. http://gallica.bnf.fr/ark:/12148/bpt6k34894x/f222.tableDesMatieres.
- ↑ Ruff, O.; Keim, R. (1931). "Fluorierung von Verbindungen des Kohlenstoffs (Benzol und Tetrachlormethan mit Jod-5-fluorid, sowie Tetrachlormethan mit Fluor) [Fluoridation of Carbon Compounds (Benzene and Tetrachlormethane with Iodine-5-Fluoride, and Tetrachloromethane with Fluorine)]" (in German). Zeitschrift für Anorganische und Allgemeine Chemie 201 (1): 245–258. doi:10.1002/zaac.19312010122.
- ↑ Ruff, O.; Keim, R. (1930). "Das Jod-7-fluorid [The iodine-7-fluoride]" (in German). Zeitschrift für anorganische und allgemeine Chemie 193 (1): 176–186. doi:10.1002/zaac.19301930117.
- ↑ Holloway, John H.; Mitchell, S. J. (1971). "Preparation and Crystal Structure of Osmium Pentafluoride". Journal of the Chemical Society: 2789–94. doi:10.1039/J19710002789.
- ↑ Stevens, T. E. (1966). "Rearrangement of Amides with Iodine Pentafluoride". Journal of Organic Chemistry 31 (6): 2025–2026. doi:10.1021/jo01344a539.
Further reading
- Lord, R. C.; Lynch, M. A.; Schumb, W. C.; Slowinski, E. J. (1950). "The Vibrational Spectra and Structures of Iodine Pentafluoride and Heptafluoride". Journal of the American Chemical Society 72 (1): 522–527. doi:10.1021/ja01157a135.
- Rogers, M. T.; Speirs, J. L.; Thompson, H. B.; Panish, M. B. (1954). "Iodine Pentafluoride, Freezing and Boiling Point, Heat of Vaporization and Vapor Pressure-Temperature Relations". Journal of the American Chemical Society 76 (19): 4843–4844. doi:10.1021/ja01648a022.
- Rogers, M. T.; Thompson, H. B.; Speirs, J. L. (1954). "Dielectric Constants of Liquid Chlorine Trifluoride and Iodine Pentafluoride". Journal of the American Chemical Society 76 (19): 4841–4843. doi:10.1021/ja01648a021.
- Booth, H. S.; Pinkston, J. T. Jr. (1947). "The Halogen Fluorides". Chemical Reviews 41 (3): 421–439. doi:10.1021/cr60130a001. PMID 18895518.
- Hetherington, G.; Robinson, P.L. (1956). "The Viscosities of Iodine Pentafluoride and Ditellurium Decafluoride". Journal of the Chemical Society (Resumed): 3681. doi:10.1039/jr9560003674. ISSN 0368-1769.
External links
- WebBook page for IF5
- National Pollutant Inventory - fluoride and compounds fact sheet
- web elements listing
 |