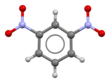
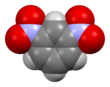
Chemistry:1,3-Dinitrobenzene
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3-Dinitrobenzene | |||
| Other names
meta-dinitrobenzene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1597 3443 | ||
| |||
| |||
| Properties | |||
| C6H4N2O4 | |||
| Molar mass | 168.108 g·mol−1 | ||
| Appearance | yellow solid | ||
| Density | 1.575 g/cm3 | ||
| Melting point | 89.6 °C (193.3 °F; 362.8 K) | ||
| Boiling point | 297 °C (567 °F; 570 K) | ||
| Hazards | |||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
| H300, H310, H330, H373, H410 | |||
| P260, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P304+340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233, P405, P501 | |||
| Flash point | 149 °C (300 °F; 422 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,3-Dinitrobenzene is one of three isomers of dinitrobenzene, with the formula C6H4(NO2)2. It is one of three isomers of dinitrobenzene. The compound is a yellow solid that is soluble in organic solvents.
Preparation
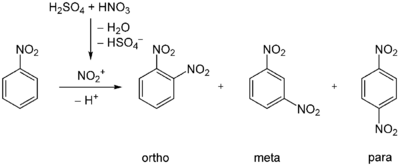
1,3-Dinitrobenzene is accessible by nitration of nitrobenzene. The reaction proceeds under acid catalysis using sulfuric acid. The directing effect of the nitro group of nitrobenzene leads to 93% of the product resulting from nitration at the meta-position. The ortho- and para-products occur in only 6% and 1%, respectively.[1]
Reactions
Reduction of 1,3-dinitrobenzene with sodium sulfide in aqueous solution leads to 3-nitroaniline. Further reduction with iron and hydrochloric acid (HCl) gives m-phenylenediamine.[2]
1,3-Dinitrobenzene can be nitrated to 1,3,5-trinitrobenzene with nitronium tetrafluoroborate in fluorosulfuric acid at 150 °C.[3][4]
References
- ↑ Joachim Buddrus (2003). Grundlagen der organischen Chemie (3 ed.). Berlin: de Gruyter. p. 360. ISBN 3-11-014683-5.
- ↑ Hans Beyer and Wolfgang Walter (1981). Lehrbuch der Organischen Chemie (19 ed.). Stuttgart: S. Hirzel Verlag. pp. 536, 542. ISBN 3-7776-0356-2.
- ↑ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. p. 688. ISBN 978-0-471-72091-1.
- ↑ Olah, George A.; Lin, Henry C. (1974). "Synthetic Methods and Reactions; XI1. A Convenient Direct Preparation of 1,3,5-Trinitrobenzene from m-Dinitrobenzene by Nitration with Nitronium Tetrafluoroborate in Fluorosulfuric Acid Solution". Synthesis 1974 (6): 444–445. doi:10.1055/s-1974-23344.
 |