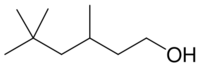
Chemistry:3,5,5-Trimethyl-1-hexanol
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3,5,5-Trimethyl-1-hexanol
| |
| Other names
Nonylol
Trimethylhexanol [1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | 3,5,5-trimethyl-1-hexanol |
| |
| |
| Properties | |
| C9H20O | |
| Molar mass | 144.258 g·mol−1 |
| Appearance | Clear liquid |
| Odor | herbaceous, plant-like [2] |
| Density | 0.824 g/mL |
| Melting point | -70 °C |
| Boiling point | 194.0 °C |
| 0.45 g/L | |
| Solubility | Soluble in alcohol, acetone, ester |
| Vapor pressure | 0.2 torr [1] |
Henry's law
constant (kH) |
4.12·10−5 atm m3 / mol [1] |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H227, H315, H319, H373, H411 | |
| P210, P260, P264, P273, P280, P302+352, P305+351+338, P314, P332+313, P337+313, P362, P370+378, P391, P403+235, P501 | |
| Flash point | 80°C |
| Related compounds | |
Related compounds
|
Isononyl alcohol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3,5,5-Trimethyl-1-hexanol is a nine carbon primary alcohol, and it makes up the mixture isononanol along with isononyl alcohol. It is used for fragrance in many toiletries and household cleaning products. Between one and ten metric tonnes are produced every year for use as a fragrance.[1]
References
- ↑ 1.0 1.1 1.2 1.3 McGinty, D.; Scognamiglio, J.; Letizia, C. S.; Api, A. M. (2010-07-01). "Fragrance material review on 3,5,5-trimethyl-1-hexanol" (in en). Food and Chemical Toxicology. A Safety Assessment of Saturated Branched Chain Alcohols when used as Fragrance Ingredients 48: S47–S50. doi:10.1016/j.fct.2010.05.026. ISSN 0278-6915. PMID 20659634. https://www.sciencedirect.com/science/article/pii/S0278691510003029.
- ↑ Lee, G. H.; Shin, Y.; Oh, M. J. (2008). "Aroma-active components of Lycii fructus (kukija)". Journal of Food Science 73 (6): C500–505. doi:10.1111/j.1750-3841.2008.00851.x. ISSN 0022-1147. PMID 19241541. https://pubmed.ncbi.nlm.nih.gov/19241541/.

