Chemistry:4-Vinylcyclohexene
From HandWiki
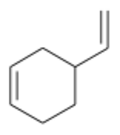
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Ethenylcyclohex-1-ene | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C8H12 | |
| Molar mass | 108.184 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.8299 g/cm3 at 20°C |
| Melting point | −108.9 °C (−164.0 °F; 164.2 K) |
| Boiling point | 128.9 °C (264.0 °F; 402.0 K) |
| 0.05 g/L[1] | |
| Solubility | soluble in benzene, diethyl ether, petroleum ether |
| Vapor pressure | 2 kPa |
Refractive index (nD)
|
1.4639 (20 °C) |
| Hazards | |
| Safety data sheet | Oxford University |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H351 | |
| P201, P202, P281, P308+313, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 21.2 °C (70.2 °F; 294.3 K)[3] |
| 269 °C (516 °F; 542 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2563 mg/kg (oral, rat)[2] |
| Related compounds | |
Related compounds
|
Buta-1,3-diene Cyclohexene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
4-Vinylcyclohexene is an organic compound consisting of a vinyl group attached to the 4-position of the cyclohexene ring. It is a colorless liquid. Although chiral, it is used mainly as the racemate. It is a precursor to vinylcyclohexene dioxide.[4]
Production
It is produced by buta-1,3-diene dimerization in a Diels-Alder reaction.[5][4] The reaction is conducted at 110 - 425 °C at pressures of 1.3 - 100 MPa in the presence of a catalyst. A mixture of silicon carbide and salts of copper or chromium comprises the catalyst. A competing product is 1,5-cyclooctadiene.
Safety
4-Vinylcyclohexene is classified as a Group 2B carcinogen by the IARC ("possibly carcinogenic to humans").[3]
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 8–111. ISBN 0-8493-0594-2.
- ↑ "Safety (MSDS) data for 4-vinylcyclohexene". Oxford University. http://msds.chem.ox.ac.uk/VI/4-vinylcyclohexene.html.
- ↑ 3.0 3.1 "4-Vinylcyclohexene". IARC. http://monographs.iarc.fr/ENG/Monographs/vol60/mono60-13.pdf.
- ↑ 4.0 4.1 Schiffer, Thomas; Oenbrink, Georg. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_205.pub2.
- ↑ Wittcoff, Harold; Reuben, B. G.; Plotkin, Jeffrey S. (1998). Industrial Organic Chemicals (2 ed.). Wiley-Interscience. pp. 236–7. ISBN 978-0-471-44385-8. https://books.google.com/books?id=4KHzc-nYPNsC&dq=4-vinylcyclohexene+diels-alder&pg=PA236. Retrieved 2009-04-19.
 |



