Chemistry:Vadadustat
 | |
| Clinical data | |
|---|---|
| Trade names | Vafseo |
| Other names | AKB-6548, PG-1016548 |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| Chemical and physical data | |
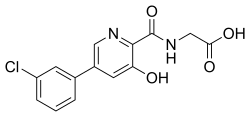
| Formula | C14H11ClN2O4 |
| Molar mass | 306.70 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vadadustat, sold under the brand name Vafseo is a medication used for the treatment of symptomatic anemia associated with chronic kidney disease.[2]
The most common side effects include thromboembolic events (problems due to the formation of blood clots in the blood vessels), diarrhea, and hypertension (high blood pressure).[2]
Vadadustat was approved for medical use in the European Union in April 2023.[2]
Medical uses
Vadadustat is indicated for the treatment of symptomatic anemia associated with chronic kidney disease in adults on chronic maintenance dialysis.[2]
Society and culture
Legal status
On 23 February 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Vafseo, intended for the treatment of symptomatic anemia in adults with chronic kidney disease who are on chronic dialysis.[3] The applicant for this medicinal product is Akebia Europe Limited.[3] Vadadustat was approved for medical use in the European Union in April 2023.[2]
Research
Vadadustat is in phase III clinical trials for the treatment of anemia caused by chronic kidney disease.[4][5][6][7][8]
References
- ↑ 1.0 1.1 https://www.tga.gov.au/resources/auspmd/vafseo
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Vafseo EPAR". 31 May 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/vafseo. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ 3.0 3.1 "Vafseo: Pending EC decision". European Medicines Agency (EMA). 24 February 2023. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/vafseo. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ↑ "Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease". Kidney International 90 (5): 1115–1122. November 2016. doi:10.1016/j.kint.2016.07.019. PMID 27650732.
- ↑ "Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD". American Journal of Kidney Diseases 69 (6): 815–826. June 2017. doi:10.1053/j.ajkd.2016.12.011. PMID 28242135.
- ↑ "Clinical Trial of Vadadustat in Patients with Anemia Secondary to Stage 3 or 4 Chronic Kidney Disease". American Journal of Nephrology 45 (5): 380–388. 2017. doi:10.1159/000464476. PMID 28343225.
- ↑ "Safety and Efficacy of Vadadustat for Anemia in Patients Undergoing Dialysis". The New England Journal of Medicine 384 (17): 1601–1612. April 2021. doi:10.1056/NEJMoa2025956. PMID 33913638.
- ↑ "Vadadustat in Patients with Anemia and Non-Dialysis-Dependent CKD". The New England Journal of Medicine 384 (17): 1589–1600. April 2021. doi:10.1056/NEJMoa2035938. PMID 33913637.
 |

