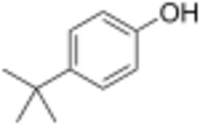
Chemistry:4-tert-Butylphenol
| |
| Names | |
|---|---|
| IUPAC name
4-tert-Butylphenol
| |
| Other names
p-tert-Butylphenol; Butylphen, Para tertiary butylphenol
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.221 g·mol−1 |
| Appearance | white solid |
| Density | 0.908 g/cm3 (20 °C)[1] |
| Melting point | 99.5 °C (211.1 °F; 372.6 K)[1] |
| Boiling point | 239.8 °C (463.6 °F; 513.0 K)[1] |
| 0.6 g/L (20 °C)[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
4-tert-Butylphenol is an organic compound with the formula (CH3)3CC6H4OH. It is one of three isomeric tert-butyl phenols. It is a white solid with a distinct phenolic odor. It dissolves in basic water.[2]
Manufacture
It can be prepared by acid-catalyzed alkylation of phenol with isobutene, 2-tert-butylphenol being the major side product. It can be produced by diverse transalkylation reactions.
Uses
It major uses are in the production of epoxy resins and curing agents and also in polycarbonate resins. It has also found use in the production of phenolic resins. Another use is in the production of para tertiary butylphenol formaldehyde resin. It has also found use as a plasticizer.
Bisphenol A is difunctional and used to produce epoxy resin and polycarbonate. 4-tert-Butylphenol is monofunctional and so in polymer science terms, bisphenol A is a polymer chain extender but 4-tert-butylphenol is a chain stopper or sometimes called endcapper. It is thus use to control molecular weight by limiting chain growth.
4-tert-Butylphenol has an OH group and so it may be reacted with epichlorohydrin and sodium hydroxide to produce the glycidyl ether which is used in epoxy resin chemistry. This molecule has the CAS Registry number of 3101-60-8.[3][4]
Laboratory reactions
Hydrogenation gives trans-4-tert-butylcyclohexanol.[5]
Controlled condensation with formaldehyde gives calixarenes.[6]
Safety
OSHA records show it as an irritant and has developed suggested test methods for its detection.[7] A European Union risk assessment report into the molecule dated May 2006 suggested it had potential depigmenting properties and was a potential endocrine disruptor. This was done under its EINECS number of 202-679-0.[8] Other papers have been published showing similar findings.[9]
References
- ↑ 1.0 1.1 1.2 1.3 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ "CAS Common Chemistry". https://commonchemistry.cas.org/detail?cas_rn=3101-60-8.
- ↑ "4-tert-butylphenyl glycidyl ether" (in en). https://www.wikidata.org/wiki/Q27276612.
- ↑ Eliel, E. L.; Martin, R. J. L.; Nasipuri, D. (1967). "trans-4-t-BUTYLCYCLOHEXANOL". Organic Syntheses 47: 16. doi:10.15227/orgsyn.047.0016. http://orgsyn.org/demo.aspx?prep=CV5P0175.
- ↑ C. D. Gutsche, M. Iqbal (1990). "p-tert-Butylcalix[4]arene". Organic Syntheses 68: 234. doi:10.15227/orgsyn.068.0234.
- ↑ "Sampling and Analytical Methods: p-tert-Butylphenol, PV2085". https://www.osha.gov/dts/sltc/methods/partial/t-pv2085-01-9203-ch/t-pv2085-01-9203-ch.html.
- ↑ European Union Risk Assessment report P-TERTIARY-BUTYLPHENOL May 2006 carried out in Norway
- ↑ Meier, Sonnich; Andersen, Thorny Cesilie; Lind-Larsen, Kristin; Svardal, Asbjørn; Holmsen, Holm (April 2007). "Effects of alkylphenols on glycerophospholipids and cholesterol in liver and brain from female Atlantic cod (Gadus morhua)". Comparative Biochemistry and Physiology. Toxicology & Pharmacology 145 (3): 420–430. doi:10.1016/j.cbpc.2007.01.012. ISSN 1532-0456. PMID 17344102. https://pubmed.ncbi.nlm.nih.gov/17344102.
 |

