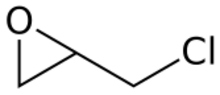
Chemistry:Epichlorohydrin
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-(Chloromethyl)oxirane | |||
| Other names
(Chloromethyl)oxirane
Epichlorohydrin 1-Chloro-2,3-epoxypropane γ-Chloropropylene oxide Glycidyl chloride ECH | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 79785 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 164180 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2023 | ||
| |||
| |||
| Properties | |||
| C3H5ClO | |||
| Molar mass | 92.52 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | garlic or chloroform-like | ||
| Density | 1.1812 g/cm3 | ||
| Melting point | −25.6 °C (−14.1 °F; 247.6 K) | ||
| Boiling point | 117.9 °C (244.2 °F; 391.0 K) | ||
| 7% (20°C)[2] | |||
| Vapor pressure | 13 mmHg (20°C)[2] | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| GHS pictograms |     
| ||
| GHS Signal word | Danger | ||
| H226, H301, H311, H314, H317, H331, H350 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P272, P280, P281, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P308+313, P310, P311 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 32 °C (90 °F; 305 K) | ||
| Explosive limits | 3.8–21%[2] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
3617 ppm (rat, 1 hr) 2165 ppm (rat, 1 hr) 250 ppm (rat, 8 hr) 244 ppm (rat, 8 hr) 360 ppm (rat, 6 hr)[3] | ||
LCLo (lowest published)
|
250 ppm (rat, 4 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 5 ppm (19 mg/m3) [skin][2] | ||
REL (Recommended)
|
Carcinogen[2] | ||
IDLH (Immediate danger)
|
Ca [75 ppm][2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents.[4] It is a chiral molecule generally existing as a racemic mixture of right-handed and left-handed enantiomers. Epichlorohydrin is a highly reactive electrophilic compound and is used in the production of glycerol, plastics, epoxy glues and resins, epoxy diluents and elastomers.
Production
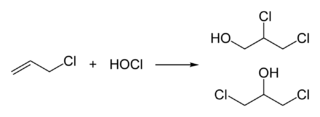
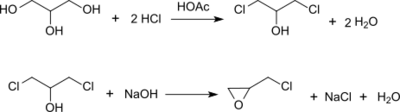
Epichlorohydrin is traditionally manufactured from allyl chloride in two steps, beginning with the addition of hypochlorous acid, which affords a mixture of two isomeric alcohols:[5][6]
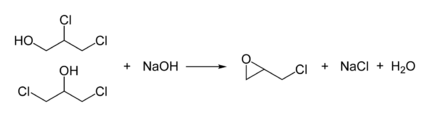
In the second step, this mixture is treated with base to give the epoxide:
In this way, more than 800,000 tons (1997) of epichlorohydrin are produced annually.[7]
Glycerol routes
Epichlorohydrin was first described in 1848 by Marcellin Berthelot. The compound was isolated during studies on reactions between glycerol and gaseous hydrogen chloride.[8]
Reminiscent of Berthelot's experiment, glycerol-to-epichlorohydrin (GTE) plants have been commercialized. This technology capitalizes on the availability of cheap glycerol from biofuels processing.[9] In the process developed by Dow Chemical, glycerol undergoes two substitution reactions when treated with hydrogen chloride in the presence of a carboxylic acid catalyst. This is the same intermediate formed in the allyl chloride/hypochlorous acid process, and is likewise then treated with base to form epichlorohydrin.[10]
Other routes
Routes that involve fewer chlorinated intermediates have continued to attract interest. One such process entails epoxidation of allyl chloride.[11]
Applications
Glycerol and epoxy resins synthesis
Epichlorohydrin is mainly converted to bisphenol A diglycidyl ether, a building block in the manufacture of epoxy resins.[12] It is also a precursor to monomers for other resins and polymers. Another usage is the conversion to synthetic glycerol. However, the rapid increase in biodiesel production, where glycerol is a waste product, has led to a glut of glycerol on the market, rendering this process uneconomical. Synthetic glycerol is now used only in sensitive pharmaceutical, and biotech applications where quality standards are very high.[13]
Minor and niche applications
Epichlorohydrin is a versatile precursor in the synthesis of many organic compounds. For example, it is converted to glycidyl nitrate, an energetic binder used in explosive and propellant compositions.[14] The epichlorohydrin is reacted with an alkali nitrate, such as sodium nitrate, producing glycidyl nitrate and alkali chloride. It is used as a solvent for cellulose, resins, and paints, and it has found use as an insect fumigant.[15]
Polymers made from epichlorohydrin, e.g., polyamide-epichlorohydrin resins, are used in paper reinforcement and in the food industry to manufacture tea bags, coffee filters, and sausage/salami casings as well as with water purification.[16]
An important biochemical application of epichlorohydrin is its use as crosslinking agent for the production of Sephadex size-exclusion chromatographic resins from dextrans.[17]
Safety
Epichlorohydrin is classified by several international health research agencies and groups as a probable or likely carcinogen in humans.[18][19][20] Prolonged oral consumption of high levels of epichlorohydrin could result in stomach problems and an increased risk of cancer.[21] Occupational exposure to epichlorohydrin via inhalation could result in lung irritation and an increased risk of lung cancer.[22]
References
- ↑ Merck Index, 12th Edition, 3648.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 NIOSH Pocket Guide to Chemical Hazards. "#0254". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0254.html.
- ↑ 3.0 3.1 "Epichlorohydrin". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/106898.html.
- ↑ "EPA consumer factsheet". Epa.gov. http://www.epa.gov/safewater/contaminants/dw_contamfs/epichlor.html.
- ↑ Braun, G. (1936). "Epichlorohydrin and Epybromohydrin". Organic Syntheses 16: 30. doi:10.15227/orgsyn.016.0030.
- ↑ Guenter Sienel; Robert Rieth; Kenneth T. Rowbottom. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a09_531.
- ↑ Ludger Krähling; Jürgen Krey; Gerald Jakobson; Johann Grolig; Leopold Miksche. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_425.
- ↑ Berthelot, Marcellin (1854). "Sur les combinaisons de la glycérine avec les acides et sur la synthèse des principes immédiats des graisses animaux". Ann. Chim. Phys.. Série 3 41: 216–319. http://gallicalabs.bnf.fr/ark:/12148/bpt6k34782n/f297.item. Retrieved 2015-03-02.
- ↑ Doris de Guzman (2011-01-20). "Growing glycerine-to-ECH plants". ICIS Green Chemicals. http://www.icis.com/blogs/green-chemicals/2011/01/growing-glycerine-to-ech-plant.html.
- ↑ Bell, Bruce M.; Briggs, John R.; Campbell, Robert M.; Chambers, Susanne M.; Gaarenstroom, Phil D.; Hippler, Jeffrey G.; Hook, Bruce D.; Kearns, Kenneth et al. (2008). "Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process" (full text reprint). CLEAN - Soil, Air, Water 36 (8): 657. doi:10.1002/clen.200800067. http://www.dow.com/innovation/pdf/Organic/Glycerin_Renewable_Feedstock.pdf. Retrieved 2012-03-05.
- ↑ Jun Li; Gongda Zhao; Shuang Gao; Ying Lv; Jian Li; Zuwei Xi (2006). "Epoxidation of Allyl Chloride to Epichlorohydrin by a Reversible Supported Catalyst with H2O2 under Solvent-Free Conditions". Org. Process Res. Dev. 10 (5): 876–880. doi:10.1021/op060108k.
- ↑ Pham, Ha Q.; Marks, Maurice J. (2012). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry (Weinheim: Wiley-VCH). doi:10.1002/14356007.a09_547.pub2. ISBN 978-3-527-30673-2.
- ↑ Taylor, Phil (16 October 2008). "Synthetic glycerine is back (but never really went away)!". In-Pharma Technologist. https://www.in-pharmatechnologist.com/Article/2008/10/16/Synthetic-glycerine-is-back-but-never-really-went-away.[yes|permanent dead link|dead link}}]
- ↑ Gould, R.F. Advanced Propellant Chemistry, ACS Chemistry Series 54, 1966
- ↑ "Suburban Water Testing Labs:Epichlorohydrin Fact Sheet". H2otest.com. http://www.h2otest.com/factsheets/epichlorohydrin.html.
- ↑ "Government of Canada Chemical Substances: Oxirane,(chloromethyl)-(Epichlorohydrin) CAS Registry Number 106-89-8". 13 February 2008. http://www.chemicalsubstanceschimiques.gc.ca/challenge-defi/summary-sommaire/batch-lot-2/106-89-8-eng.php.
- ↑ "GE Healthcare Life Sciences - Instructions for Sephadex Media". .gelifesciences.com. http://www5.gelifesciences.com/aptrix/upp00919.nsf/content/EE755AF81C96972FC1256EB40048417B?OpenDocument&Path=Catalog&Hometitle=Catalog&entry=1&newrel&LinkParent=C1256FC4003AED40-342B143523C3C764C12570F600421582_RelatedLinksNew-C821BEC677D8448BC1256EAE002E3030&newrel&hidesearchbox=yes&moduleid=166703.
- ↑ "EPA Integrated Risk Information System: Epichlorohydrin (CASRN 106-89-8)". http://www.epa.gov/iris/subst/0050.htm.
- ↑ "Government of Canada: Screening Assessment for Epichlorohydrin". http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=BA416AA1-1.
- ↑ "NIOSH Pocket Guide to Chemical Hazards - Epichlorohydrin". https://www.cdc.gov/niosh/npg/npgd0254.html.
- ↑ "Basic Information about Epichlorohydrin in Drinking Water". http://water.epa.gov/drink/contaminants/basicinformation/epichlorohydrin.cfm.
- ↑ "Government of Canada: Screening Assessment for Epichlorohydrin". http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=BA416AA1-1.
 |