Chemistry:Transition metal fullerene complex
A transition metal fullerene complex is a coordination complex wherein fullerene serves as a ligand. Fullerenes are typically spheroidal carbon compounds, the most prevalent being buckminsterfullerene, C60.[2]
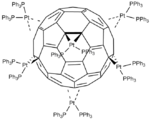
One year after it was prepared in milligram quantities in 1990,[3] C60 was shown to function as a ligand in the complex [Ph3P]2Pt(η2-C60).[4]
Since this report, a variety of transition metals and binding modes were demonstrated. Most transition metal fullerene complex are derived from C60, although other fullerenes also coordinate to metals as seen with C70Rh(H)(CO)(PPh3)2.[5]
Binding modes
As ligands, fullerenes behave similarly to electron-deficient alkenes such as tetracyanoethylene. Thus, their complexes are a subset of metal-alkene complexes. They almost always coordinate in a dihapto fashion and prefer electron-rich metal centers.[6] This binding occurs on the junction of two 6-membered rings. Hexahapto and pentahapto bonding is rarely observed.[7]
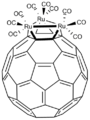
In Ru3(CO)9(C60), the fullerene binds to the triangular face of the cluster.[8]
- Illustrative Fullerene Complexes
Examples
C60 forms stable complexes of the type M(C60)(diphosphine)(CO)3 for M = Mo, W. A dirhenium complexes is known with the formula Re2(PMe3)4H8(η2:η2C60) where two of the hydrogen act as bridging ligands.[5]
Many fullerene complexes are derived from platinum metals. An unusual cationic complex features three 16e Ru centers:
- 3 Cp*Ru(MeCN)3+ + C60 → {[(Cp*Ru(MeCN)2]3C60}3+ + 3 MeCN
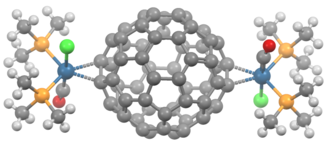
Vaska's complex forms a 1:1 adduct, and the analogous IrCl(CO)(PEt3)2 binds 200x more strongly.[2] Complexes with more than one fullerene ligand are illustrated by Ir4(CO)3(μ4-CH)(PMe3)2(μ-PMe)2(CNCH2Ph)(μ-η2:η2C60)(μ4-η1:η1:η2:η2C60). In this Ir4 cluster two fullerene ligands with multiple types of mixed binding. Platinum, palladium, and nickel form complexes of the type C60ML2 where L is a monodentate or bidentate phosphorus ligand.[5] They are prepared by displacement of weakly coordinating ligands such as ethylene:[6]
- [Ph3P]2Pt(C2H4) + C60 → [Ph3P]2Pt(η2-C60) + C2H4
In [(Et3P)2Pt]6(η2-C60), six Pt centers are bound to the fullerene.[9]
Modified fullerenes as ligands
Osmium tetraoxide adds to C60 to give, in the presence of pyridine (py), the diolate C60O2OsO2(py)2.[2]
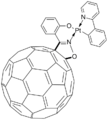
The pentaphenyl anion C60Ph5− behaves as a cyclopentadienyl ligand.[5] thumb|260 px|center|[[Ferrocene-like complex of C60Ph5−.]]
In this example, the binding of the ligand is similar to ferrocene. The anion C60(PhCH2)2Ph functions as an indenyl-like ligand.[10] Fullerenes can also be substituents on otherwise conventional ligands as seen with an isoxazoline fullerene chelating to platinum, rhenium, and iridium compounds.[11]
Ongoing research
Although no application has been commercialized. non-linear optical (NLO) materials,[12] and as supramolecular building blocks.[13]
See also
References
- ↑ Alan L. Balch; Joong W. Lee; Bruce C. Noll; Marilyn M. Olmstead (1994). "Multiple Additions of Vaska-Type Iridium Complexes to C60. Preferential Crystallization of the "Para" Double Addition Products: C60{Ir(CO)Cl(PMe3)2}2.2C6H6 and C60{Ir(CO)Cl(PEt3)2}2.C6H6". Inorg. Chem. 33: 5238–5243. doi:10.1021/ic00101a015.
- ↑ 2.0 2.1 2.2 Alan L. Balch; Marilyn M. Olmstead (1998). "Reactions of Transition Metal Complexes with Fullerenes (C60, C70, etc.) and Related Materials". Chem. Rev. 98 (6): 2123–2166. doi:10.1021/cr960040e. PMID 11848962.
- ↑ Krätschmer, W. (1990). "The infrared and ultraviolet absorption spectra of laboratory-produced carbon dust: evidence for the presence of the C60 molecule". Chemical Physics Letters 170 (2–3): 167–170. doi:10.1016/0009-2614(90)87109-5. Bibcode: 1990CPL...170..167K.
- ↑ Fagan, P.J.; Calabrese, J.C.; Malone, B. (1991). "The Chemical Nature of Buckminsterfullerene (C60) and the characterization of a platinum derivative". Science 252 (5009): 1160–1161. doi:10.1126/science.252.5009.1160. ISSN 0036-8075. Bibcode: 1991Sci...252.1160F.
- ↑ 5.0 5.1 5.2 5.3 Denisovich, L. I.; Peregudova, S. M.; Novikov, Yu. N. (2010). "Electrochemical properties of transition metal complexes with C60 and C70 fullerene ligands (review)". Russian Journal of Electrochemistry 46 (1): 1–17. doi:10.1134/S1023193510010015.
- ↑ 6.0 6.1 Spessard, p. 162
- ↑ Spessard, p. 165
- ↑ Hsu, Hsiu-Fu; Shapley, John R. (1996). "Ru3(CO)9(μ3-η2,η2,η2-C60): A Cluster Face-Capping, Arene-Like Complex of C60". J. Am. Chem. Soc. 118 (38): 9192. doi:10.1021/ja962077m.
- ↑ Fagan, P.J.; Calabrese, J.C.; Malone, B. (1991). "A multiply-substituted buckminsterfullerene (C60) with an octahedral array of platinum atoms". Journal of the American Chemical Society 113 (24): 9408–9409. doi:10.1021/ja00024a079.
- ↑ Toganoh, Motoki; Matsuo, Yutaka; Nakamura, Eiichi (2003). "Synthesis and catalytic activity of rhodium diene complexes bearing indenyl-type fullerene η5-ligand". Journal of Organometallic Chemistry 683 (2): 295–300. doi:10.1016/S0022-328X(03)00465-0.
- ↑ RamíRez-Monroy, Armando; Swager, Timothy M. (2011). "Metal Chelates Based on Isoxazoline[60]fullerenes". Organometallics 30 (9): 2464–2467. doi:10.1021/om200238a.
- ↑ Dragonetti, Claudia; Valore, Adriana; Colombo, Alessia; Righetto, Stefania; Rampinini, Giovanni; Colombo, Francesca; Rocchigiani, Luca; MacChioni, Alceo (2012). "An investigation on the second-order NLO properties of novel cationic cyclometallated Ir(III) complexes of the type [Ir(2-phenylpyridine)2(9-R-4,5-diazafluorene)]+ (R=H, fulleridene) and the related neutral complex with the new 9-fulleriden-4-monoazafluorene ligand". Inorganica Chimica Acta 382: 72–78. doi:10.1016/j.ica.2011.10.018.
- ↑ Santos, Leandro J.; Carvalhoda-Silva, Dayse; Rebouças, Júlio S.; Alves, Marcos R.A.; Idemori, Ynara M.; Matencio, Tulio; Freitas, Rossimiriam P. (2011). "Synthesis of new porphyrin/fullerene supramolecular assemblies: A spectroscopic and electrochemical investigation of their coordination equilibrium in solution". Tetrahedron 67: 228–235. doi:10.1016/j.tet.2010.10.066. http://www.locus.ufv.br/handle/123456789/21613.
Bibliography
- Spessard, Gary; Miessler, Gary (2010). Organometallic Chemistry ISBN:0195330994
 |