Chemistry:Gilman reagent
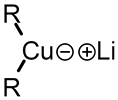
Use in organic chemistry

These reagents are useful because, unlike related Grignard reagents and organolithium reagents, they react with organic halides to replace the halide group with an R group (the Corey–House reaction). Such displacement reactions allow for the synthesis of complex products from simple building blocks.[1][2] Lewis acids can be used to modify the reagent.[2]
History
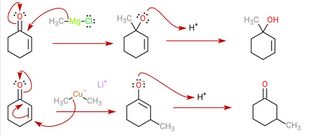
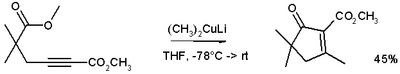
These reagents were discovered by Henry Gilman and coworkers.[3] Lithium dimethylcopper (CH3)2CuLi can be prepared by adding copper(I) iodide to methyllithium in tetrahydrofuran at −78 °C. In the reaction depicted below,[4] the Gilman reagent is a methylating reagent reacting with an alkyne in a conjugate addition, and the ester group forms a cyclic enone.
Structure
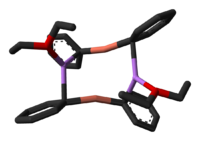
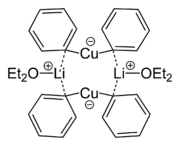
Lithium dimethylcuprate exists as a dimer in diethyl ether forming an 8-membered ring. Similarly, lithium diphenylcuprate crystallizes as a dimeric etherate, [{Li(OEt
2)}(CuPh
2)]
2.[5]
If the Li+ ions are complexed with the crown ether 12-crown-4, the resulting diorganylcuprate anions adopt a linear coordination geometry at copper.[6]
For the 'higher order cyanocuprate' Li2CuCN(CH3)2, Lipshutz and coworkers have claimed that the cyanide ligand is coordinated to Li and π-bound to Cu.[7] However, the existence of 'mixed higher order organocuprates' has been disputed by Bertz and coworkers, who rejoined that the cyano ligand is actually bound solely to the lithium atom, and that such a structure could still explain the enhanced reactivity of cuprate prepared from CuCN.[8][9] To date, no crystallographic evidence for the existence of 'mixed higher order cuprates' ([R2CuX]2–, X ≠ R) has been obtained. On the other hand, a homoleptic higher order cuprate in the form of a [Ph3Cu]2– moiety has been observed in Li3Cu2Ph5(SMe2)4, prepared by Olmstead and Power.[10]
Mixed cuprates
More useful generally than the Gilman reagents are the so-called mixed cuprates with the formula [RCuX]− and [R2CuX]2− (see above for the controversy over existence of the latter). Such compounds are often prepared by the addition of the organolithium reagent to copper(I) halides and cyanide. These mixed cuprates are more stable and more readily purified.[11] One problem addressed by mixed cuprates is the economical use of the alkyl group. Thus, in some applications, the mixed cuprate with the formula Li2[Cu(2-thienyl)(CN)R] is prepared by combining thienyllithium and cuprous cyanide followed by the organic group to be transferred. In this higher order mixed cuprate, both the cyanide and thienyl groups do not transfer, only the R group does.[12]
See also
References
- ↑ J. F. Normant (1972). "Organocopper(I) Compounds and Organocuprates in Synthesis". Synthesis 1972 (2): 63–80. doi:10.1055/s-1972-21833.
- ↑ 2.0 2.1 Woodward, Simon (2000-01-01). "Decoding the 'black box' reactivity that is organocuprate conjugate addition chemistry" (in en). Chemical Society Reviews 29 (6): 393–401. doi:10.1039/B002690P. ISSN 1460-4744. https://pubs.rsc.org/en/content/articlelanding/2000/cs/b002690p.
- ↑ Henry Gilman; Reuben G. Jones; L. A. Woods (1952). "The Preparation of Methylcopper and some Observations on the Decomposition of Organocopper Compounds". Journal of Organic Chemistry 17 (12): 1630–1634. doi:10.1021/jo50012a009.
- ↑ Modern Organocopper Chemistry, N. Krause Ed. Wiley-VCH, 2002.
- ↑ N. P. Lorenzen; E. Weiss (1990). "Synthesis and Structure of a Dimeric Lithium Diphenylcuprate:[{Li(OEt)2}(CuPh2)]2". Angew. Chem. Int. Ed. 29 (3): 300–302. doi:10.1002/anie.199003001.
- ↑ H. Hope; M. M. Olmstead; P. P. Power; J. Sandell; X. Xu (1985). "Isolation and x-ray crystal structures of the mononuclear cuprates [CuMe2]−, [CuPh2]−, and [Cu(Br)CH(SiMe3)2]−". Journal of the American Chemical Society 107 (14): 4337–4338. doi:10.1021/ja00300a047. Bibcode: 1985JAChS.107.4337H.
- ↑ Bruce H. Lipshutz; Brian James (1994). "New 1H and 13C NMR Spectral Data on "Higher Order" Cyanocuprates. If the Cyano Ligand Is Not On Copper, Then Where Is It?". J. Org. Chem. 59 (25): 7585–7587. doi:10.1021/jo00104a009.
- ↑ Bertz, Steven H.; Miao, Guobin; Eriksson, Magnus (1996-01-01). "It's on lithium! an answer to the recent communication which asked the question: ‘if the cyano ligand is not on copper, then where is it?’" (in en). Chemical Communications (7): 815–816. doi:10.1039/CC9960000815. ISSN 1364-548X. https://pubs.rsc.org/en/content/articlelanding/1996/cc/cc9960000815.
- ↑ Bertz, Steven H. (2002-05-01). "New copper chemistry. 17. Higher-order cyanocuprates: are they real?" (in EN). doi:10.1021/ja00166a046. https://pubs.acs.org/doi/pdf/10.1021/ja00166a046.
- ↑ Olmstead, Marilyn M.; Power, Philip P. (1989-05-01). "Structural characterization of a higher order cuprate: x-ray crystal structure of [Li3Cu2Ph5(SMe2)4"]. Journal of the American Chemical Society 111 (11): 4135–4136. doi:10.1021/ja00193a075. ISSN 0002-7863. https://pubs.acs.org/doi/10.1021/ja00193a075.
- ↑ Steven H. Bertz, Edward H. Fairchild, Karl Dieter, "Copper(I) Cyanide" in Encyclopedia of Reagents for Organic Synthesis 2005, John Wiley & Sons. doi:10.1002/047084289X.rc224.pub2
- ↑ Bruce H. Lipshutz, Robert Moretti, Robert Crow "Mixed Higher-order Cyanocuprate-induced Epoxide Openings: 1-Benzyloxy-4-penten-2-ol" Org. Synth. 1990, volume 69, pp. 80. doi:10.15227/orgsyn.069.0080
 |