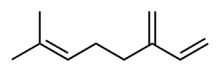
Chemistry:Myrcene
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
7-Methyl-3-methylideneocta-1,6-diene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.238 g·mol−1 |
| Density | 0.794 g/cm3 |
| Melting point | < −10 °C (14 °F; 263 K) |
| Boiling point | 166 to 168 °C (331 to 334 °F; 439 to 441 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Myrcene, or β-myrcene, is a monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from Myrcia, from which it gets its name. It is an intermediate in the production of several fragrances. α-Myrcene is the name for the isomer 2-methyl-6-methylene-1,7-octadiene, which has not been found in nature.[3]
Production
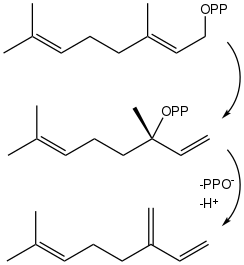
Myrcene is often produced commercially by the pyrolysis (400 °C) of β-pinene, which is obtained from turpentine.[3] It is rarely obtained directly from plants.[4]
Plants biosynthesize myrcene via geranyl pyrophosphate (GPP), which isomerizes into linalyl pyrophosphate. The release of the pyrophosphate (OPP) and a proton completes the conversion.[5]
Occurrence
It could in principle be extracted from any number of plants, such as verbena or wild thyme,[4] the leaves of which contain up to 40% by weight of myrcene.[citation needed] Many other plants contain myrcene, sometimes in substantial amounts.[3] Some of these include cannabis,[6] hops, Houttuynia, lemon grass, mango, Myrcia, West Indian bay tree, and cardamom.[7]
Of the several terpenes extracted from Humulus lupulus (hops), the largest monoterpenes fraction is β-myrcene. One study of the chemical composition of the fragrance of Cannabis sativa found β-myrcene to compose between 29.4% and 65.8% of the steam-distilled essential oil for the set of fiber and drug strains tested.[8] Additionally, myrcene is thought to be the predominant terpene found in modern cannabis cultivars within North America. Interestingly, photo-oxidation of myrcene has been shown to rearrange the molecule into a novel terpene known as "hashishene" which is named for its abundance in hashish.[9]
It is found in the South African Adenandra villosa (50%).[10] & Brazilian Schinus molle (40%)[11] Myrcene is also found in Myrcia cuprea petitgrain (up to 48%),[12] bay leaf, juniper berry, cannabis, and hops.[3][13]
Use in fragrance and flavor industries
Myrcene is an intermediate used in the perfumery industry. It has a pleasant odor but is rarely used directly.[3] It is also unstable in air, tending to polymerize. Samples are stabilized by the addition of alkylphenols or tocopherol. It is thus more highly valued as an intermediate for the preparation of flavor and fragrance chemicals, such as menthol, citral, citronellol, citronellal, geraniol, nerol, and linalool.[4] Myrcene is converted to myrcenol, another fragrance found in lavender, via hydroamination of the 1,3-diene by diethylamine followed by hydrolysis and palladium-catalyzed removal of the amine.[14]
Both myrcene and myrcenol undergo Diels-Alder reactions with several dienophiles such as acrolein to give cyclohexene derivatives that are also useful fragrances, for instance Lyral.[2]
Myrcene also contributes a peppery and balsam aroma in beer.[15][16]
As of October 2018, the U.S. FDA withdrew authorization for the use of myrcene as a synthetic flavoring substance for use in food, without regard to its continuing stance that this substance does not pose a risk to public health under the conditions of its intended use.[17]
Health and safety
In 2015, beta-myrcene was added to California's Prop 65 list of chemicals known to the state of California to cause cancer or reproductive harm.[18]
See also
- Perfume allergy
References
- ↑ Merck Index, 11th Edition, 6243
- ↑ 2.0 2.1 Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. (2002). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_141. ISBN 3-527-30673-0.
- ↑ 3.0 3.1 3.2 3.3 3.4 Behr, A.; Johnen, L. (2009). "Myrcene as a Natural Base Chemical in Sustainable Chemistry: A Critical Review". ChemSusChem 2 (12): 1072–1095. doi:10.1002/cssc.200900186. PMID 20013989.
- ↑ 4.0 4.1 4.2 M. Eggersdorfer (2005). "Terpenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_205. ISBN 3-527-30673-0.
- ↑ Dewick, Paul M. (2002). Medicinal Natural Products: A Biosynthetic Approach. New York: John Wiley and Sons, Ltd.. p. 174. ISBN 0-471-49641-3. https://books.google.com/books?id=A4zptjOJfKQC.
- ↑ Booth, Judith K.; Page, Jonathan E.; Bohlmann, Jörg (29 March 2017). Hamberger, Björn. ed. "Terpene synthases from Cannabis sativa". PLOS ONE 12 (3): e0173911. doi:10.1371/journal.pone.0173911. ISSN 1932-6203. PMID 28355238. Bibcode: 2017PLoSO..1273911B.
- ↑ Marongiu, B; Piras, A; Porcedda, S (2004). "Comparative analysis of the oil and supercritical CO2 extract of Elettaria cardamomum (L.) Maton". Journal of Agricultural and Food Chemistry 52 (20): 6278–82. doi:10.1021/jf034819i. PMID 15453700.
- ↑ "Essential oil of Cannabis sativa L. strains". http://druglibrary.net/olsen/HEMP/IHA/jiha4208.html.
- ↑ "Myrcene Terpene for Extracts, vape and concentrates". https://www.elevationterpenes.com/products/myrcene-terpene-for-extracts-vape-and-concentrates.
- ↑ Baser, K. H. C.; Demirci, B.; Ozek, T.; Viljoen, A. M.; Victor, J. E. (2006). "Composition of the essential oils of two Adenandra species from South Africa". Journal of Essential Oil Research. https://agris.fao.org/agris-search/search.do?recordID=US201301099695.
- ↑ Dannenberg, Guilherme da Silva; Funck, Graciele Daiana; Silva, Wladimir Padilha da; Fiorentini, Ângela Maria (2019). "Essential oil from pink pepper (Schinus terebinthifolius Raddi): Chemical composition, antibacterial activity and mechanism of action". Food Control 95: 115–120. doi:10.1016/j.foodcont.2018.07.034.
- ↑ Zoghbi, M das Graças B.; Andrade, Eloisa Helena A.; Da Silva, Milton Helio L.; Carreira, L. M. M.; Maia, J. G. S. (2003). "Essential oils from three Myrcia species". Flavour and Fragrance Journal 18 (5): 421–424. doi:10.1002/ffj.1242.
- ↑ Chyau, C.-C.; Mau, J.-L.; Wu, C.-M. (1996). "Characteristics of the Steam-Distilled Oil and Carbon Dioxide Extract of Zanthoxylum simulans Fruits". Journal of Agricultural and Food Chemistry 44 (4): 1096–1099. doi:10.1021/jf950577d.
- ↑ Kunihiko Takabe; Takao Katagiri; Juntaro Tanaka; Tsutomu Fujita; Shoji Watanabe; Kyoichi Suga (1989). "Addition of Dialkylamines to Myrcene: N,N-Diethylgeranylamine". Org. Synth. 67: 44. doi:10.15227/orgsyn.067.0044.
- ↑ Inui, T; Tsuchiya, F; Ishimaru, M; Oka, K; Komura, H (2013). "Different beers with different hops. Relevant compounds for their aroma characteristics". Journal of Agricultural and Food Chemistry 61 (20): 4758–64. doi:10.1021/jf3053737. PMID 23627300.
- ↑ Vázquez Araújo, L.; Rodríguez Solana, R; Cortés Diéguez, S. M.; Domínguez, J. M. (2013). "Use of hydrodistillation and headspace solid-phase microextraction to characterize the volatile composition of different hop cultivars". Journal of the Science of Food and Agriculture 93 (10): 2568–74. doi:10.1002/jsfa.6078. PMID 23483584.
- ↑ 83 FR 50490
- ↑ "Proposition 65". OEHHA - California Office of Environmental Health Hazard Assessment. https://oehha.ca.gov/proposition-65/chemicals/beta-myrcene.
 |

