Chemistry:Pyrophosphate

| |
 Phosphorus, P Oxygen, O | |
| Names | |
|---|---|
| IUPAC name
Diphosphate
| |
| Systematic IUPAC name
Dipolyphosphate | |
| Other names
Pyrophosphate
Phosphonatophosphate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| 26938 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| P 2O4− 7 | |
| Molar mass | 173.941 g·mol−1 |
| Conjugate acid | Pyrophosphoric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P–O–P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate (Na
2H
2P
2O
7) and tetrasodium pyrophosphate (Na
4P
2O
7), among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P–O–P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.
Acidity
Pyrophosphoric acid is a tetraprotic acid, with four distinct pKa's:[1]
- H
4P
2O
7 ⇌ [H
3P
2O
7]−
+ H+
, pKa1 = 0.85 - [H
3P
2O
7]−
⇌ [H
2P
2O
7]2− + H+
, pKa2 = 1.96 - [H
2P
2O
7]2− ⇌ [HP
2O
7]3− + H+
, pKa3 = 6.60 - [HP
2O
7]3− ⇌ [P
2O
7]4− + H+
, pKa4 = 9.41
The pKa's occur in two distinct ranges because deprotonations occur on separate phosphate groups. For comparison with the pKa's for phosphoric acid are 2.14, 7.20, and 12.37.
At physiological pH's, pyrophosphate exists as a mixture of doubly and singly protonated forms.
Preparation
Disodium pyrophosphate is prepared by thermal condensation of sodium dihydrogen phosphate or by partial deprotonation of pyrophosphoric acid.[2]
Pyrophosphates are generally white or colorless. The alkali metal salts are water-soluble.[3] They are good complexing agents for metal ions (such as calcium and many transition metals) and have many uses in industrial chemistry. Pyrophosphate is the first member of an entire series of polyphosphates.[4]
In biochemistry
The anion P
2O4−
7 is abbreviated PPi, standing for inorganic pyrophosphate. It is formed by the hydrolysis of ATP into AMP in cells.
- ATP → AMP + PPi
For example, when a nucleotide is incorporated into a growing DNA or RNA strand by a polymerase, pyrophosphate (PPi) is released. Pyrophosphorolysis is the reverse of the polymerization reaction in which pyrophosphate reacts with the 3′-nucleosidemonophosphate (NMP or dNMP), which is removed from the oligonucleotide to release the corresponding triphosphate (dNTP from DNA, or NTP from RNA).
The pyrophosphate anion has the structure P
2O4−
7, and is an acid anhydride of phosphate. It is unstable in aqueous solution and hydrolyzes into inorganic phosphate:
- P
2O4−
7 + H
2O → 2 HPO2−
4
or in biologists' shorthand notation:
- PP
i + H
2O → 2 P
i + 2 H+
In the absence of enzymic catalysis, hydrolysis reactions of simple polyphosphates such as pyrophosphate, linear triphosphate, ADP, and ATP normally proceed extremely slowly in all but highly acidic media.[5]
(The reverse of this reaction is a method of preparing pyrophosphates by heating phosphates.)
This hydrolysis to inorganic phosphate effectively renders the cleavage of ATP to AMP and PPi irreversible, and biochemical reactions coupled to this hydrolysis are irreversible as well.
PPi occurs in synovial fluid, blood plasma, and urine at levels sufficient to block calcification and may be a natural inhibitor of hydroxyapatite formation in extracellular fluid (ECF).[6] Cells may channel intracellular PPi into ECF.[7] ANK is a nonenzymatic plasma-membrane PPi channel that supports extracellular PPi levels.[7] Defective function of the membrane PPi channel ANK is associated with low extracellular PPi and elevated intracellular PPi.[6] Ectonucleotide pyrophosphatase/phosphodiesterase (ENPP) may function to raise extracellular PPi.[7]
From the standpoint of high energy phosphate accounting, the hydrolysis of ATP to AMP and PPi requires two high-energy phosphates, as to reconstitute AMP into ATP requires two phosphorylation reactions.
- AMP + ATP → 2 ADP
- 2 ADP + 2 Pi → 2 ATP
The plasma concentration of inorganic pyrophosphate has a reference range of 0.58–3.78 µM (95% prediction interval).[8]
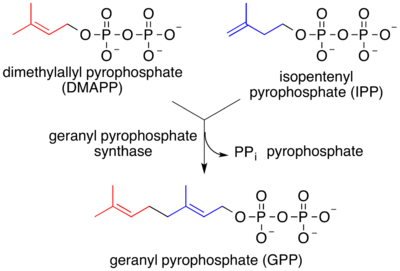
Terpenes
Isopentenyl pyrophosphate converts to geranyl pyrophosphate, the precursor to tens of thousands of terpeness and terpenoids.[9]
As a food additive
Various diphosphates are used as emulsifiers, stabilisers, acidity regulators, raising agents, sequestrants, and water retention agents in food processing.[10] They are classified in the E number scheme under E450:[11]
- E450(a): disodium dihydrogen diphosphate; trisodium diphosphate; tetrasodium diphosphate (TSPP); tetrapotassium diphosphate
- E450(b): pentasodium and pentapotassium triphosphate
- E450(c): sodium and potassium polyphosphates
In particular, various formulations of diphosphates are used to stabilize whipped cream.[12]
See also
- Adenosine monophosphate
- Adenosine diphosphate
- Adenosine triphosphate
- ATPase
- ATP hydrolysis
- ATP synthase
- Biochemistry
- Bone
- Calcium pyrophosphate
- Calcium pyrophosphate dihydrate deposition disease
- Catalysis
- DNA
- High energy phosphate
- Inorganic pyrophosphatase
- Nucleoside triphosphate
- Nucleotide
- Organophosphate
- Oxidative phosphorylation
- Phosphate
- Phosphoric acid
- Phosphoric acids and phosphates
- RNA
- Sodium pyrophosphate
- Superphosphate
- Thiamine pyrophosphate
- Tooth
- Zinc pyrophosphate
References
- ↑ Yadav, Prerna; Blacque, Olivier; Roodt, Andreas; Zelder, Felix (2021). "Induced fit activity-based sensing: A mechanistic study of pyrophosphate detection with a "flexible" Fe-salen complex". Inorganic Chemistry Frontiers 8 (19): 4313–4323. doi:10.1039/d1qi00209k. PMID 34603734.
- ↑ Bell, R. N. (1950). "Sodium Pyrophosphates (Sodium Diphosphates)". Inorganic Syntheses. Inorganic Syntheses. 3. pp. 98–101. doi:10.1002/9780470132340.ch24. ISBN 9780470132340.
- ↑ C.Michael Hogan. 2011. Phosphate. Encyclopedia of Earth. Topic ed. Andy Jorgensen. Ed.-in-Chief C.J.Cleveland. National Council for Science and the Environment. Washington DC
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ "Structure and Properties of the Condensed Phosphates. VII. Hydrolytic Degradation of Pyro- and Tripolyphosphate". J. Am. Chem. Soc. 77 (2): 287–291. Jan 1955. doi:10.1021/ja01607a011.
- ↑ 6.0 6.1 "Role of the mouse ank gene in control of tissue calcification and arthritis". Science 289 (5477): 265–70. Jul 2000. doi:10.1126/science.289.5477.265. PMID 10894769. Bibcode: 2000Sci...289..265H.
- ↑ 7.0 7.1 7.2 "PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification". Am J Pathol 158 (2): 543–54. Feb 2001. doi:10.1016/S0002-9440(10)63996-X. PMID 11159191.
- ↑ "Quantification of human plasma inorganic pyrophosphate. I. Normal values in osteoarthritis and calcium pyrophosphate dihydrate crystal deposition disease". Arthritis Rheum. 22 (8): 886–91. 1979. doi:10.1002/art.1780220812. PMID 223577.
- ↑ Eberhard Breitmaier (2006). "Hemi‐ and Monoterpenes". Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. pp. 10–23. doi:10.1002/9783527609949.ch2. ISBN 9783527609949. https://archive.org/details/terpenes00brei.
- ↑ Codex Alimentarius 1A, 2nd ed, 1995, pp. 71, 82, 91
- ↑ D. J. Jukes, Food Legislation of the UK: A Concise Guide, Elsevier, 2013, p. 60–61
- ↑ Ricardo A. Molins, Phosphates in Food, p. 115
Further reading
- "Polyphosphate in bone". Biochemistry (Moscow) 65 (3): 296–303. Mar 2000. PMID 10739471. http://protein.bio.msu.su/biokhimiya/contents/v65/pdf/bcm_0296.pdf.
External links
- Pyrophosphates at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
