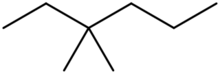
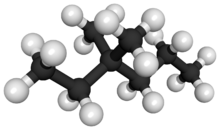
Chemistry:3,3-Dimethylhexane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,3 Dimethylhexane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 1262[1] |
| |
| |
| Properties | |
| C8H18 | |
| Molar mass | 114.232 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Odourless |
| Melting point | −126.10 °C; −194.98 °F; 147.05 K |
| Boiling point | 111.90 °C; 233.42 °F; 385.05 K |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | DANGER |
| H225, H304, H315, H336, H410 | |
| P210, P261, P273, P301+310, P331 | |
| Flash point | 7 °C (45 °F; 280 K) |
| 425 °C (797 °F; 698 K) | |
| Related compounds | |
Related alkanes
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3,3-Dimethylhexane is a colourless, odourless liquid, chemical compound in the family of hydrocarbons which has a formula of C8H18. It is an isomer of octane, where two methylene hydrogens at the third position in a hexane molecule have been replaced with two methyl groups.[2][3][4][5]
3,3-Dimethylhexane is found in various herbs and spices. It is also a constituent in the oil of osmanthus fragrans and Ginseng. 3,3-Dimethylhexane is an acyclic alkane, where there are no cycles in the structure of the molecule.[6]
It is one of the main extracts from cinnamon bark using supercritical carbon dioxide extraction, with it being 10.6% of the extracted material; it is second behind trans-cinnamaldehyde with it being 32.1% of the extracted material.[7]
Uses
3,3-dimethylhexane can be used in the production of phytochemical compounds which are effective in the removal of heavy metals, since when cyanobacteria are exposed to aliphatic compounds, or alkanes, and some heavy metals they produce phytochemical compounds which are effective in the removal of heavy metals. 3,3-dimethylhexane being both an alkane and an aliphatic compound can be used in this process.[8]
References
- ↑ PubChem. "3,3-Dimethylhexane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11233.
- ↑ "Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.008.404.
- ↑ PubChem. "3,3-Dimethylhexane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11233.
- ↑ "3,3-Dimethylhexane | C8H18 | ChemSpider". http://www.chemspider.com/Chemical-Structure.10759.html?rid=fcab38bb-cb36-4a94-b953-3575b27990da.
- ↑ "3,3-dimethylhexane (CHEBI:132182)". https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:132182.
- ↑ "T3DB: 3,3-Dimethylhexane". http://www.t3db.ca/toxins/T3D3449.
- ↑ Wang, Yang; Dai, Pei-Pei; Guo, Shan-Shan; Cao, Ju-Qin; Pang, Xue; Geng, Zhu-Feng; Sang, Yu-Li; Du, Shu-Shan (2018-08-01). "Supercritical carbon dioxide extract of Cinnamomum cassia bark: toxicity and repellency against two stored-product beetle species" (in en). Environmental Science and Pollution Research 25 (22): 22236–22243. doi:10.1007/s11356-018-2342-2. ISSN 1614-7499. PMID 29804253. Bibcode: 2018ESPR...2522236W. https://doi.org/10.1007/s11356-018-2342-2.
- ↑ Ghorbani, Elham; Nowruzi, Bahareh; Nezhadali, Masoumeh; Hekmat, Azadeh (2022-02-17). "Metal removal capability of two cyanobacterial species in autotrophic and mixotrophic mode of nutrition". BMC Microbiology 22 (1): 58. doi:10.1186/s12866-022-02471-8. ISSN 1471-2180. PMID 35176992.
 |

