Biology:Immunoglobulin D
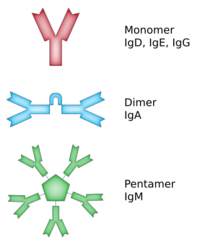
Immunoglobulin D (IgD) is an antibody isotype that makes up about 1% of proteins in the plasma membranes of immature B-lymphocytes where it is usually co-expressed with another cell surface antibody called IgM. IgD is also produced in a secreted form that is found in very small amounts in blood serum, representing 0.25% of immunoglobulins in serum. The relative molecular mass and half-life of secreted IgD is 185 kDa and 2.8 days, respectively.[1] Secreted IgD is produced as a monomeric antibody with two heavy chains of the delta (δ) class, and two Ig light chains.
Function
The function of IgD has been a puzzle in immunology since its discovery in 1964. IgD is present in species from cartilaginous fish to humans (with the possible exception of birds).[2] This nearly ubiquitous appearance in species with an adaptive immune system demonstrates that IgD may be as ancient as IgM and suggests that IgD has important immunological functions.
In B cells, the function of IgD is to signal the B cells to be activated. By being activated, B cells are ready to take part in the defense of the body as part of the immune system. During B cell differentiation, IgM is the exclusive isotype expressed by immature B cells. IgD starts to be expressed when the B cell exits the bone marrow to populate peripheral lymphoid tissues. When a B cell reaches its mature state, it co-expresses both IgM and IgD. A 2016 study by Übelhart and colleagues found that IgD signaling is only triggered by repetitive multivalent immunogens, while IgM can be triggered either by soluble monomeric or by multivalent immunogens.[3] Cδ knockout mice (mice that have been genetically altered so that they do not produce IgD) have no major B cell intrinsic defects.[4][5] IgD may have some role in allergic reactions.[citation needed]
IgD was found to bind to basophils and mast cells and activate these cells to produce antimicrobial factors to participate in respiratory immune defense in humans.[6] It also stimulates basophils to release B cell homeostatic factors. This is consistent with the reduction in the number of peripheral B cells, reduced serum IgE level and defective primary IgG1 response in IgD knockout mice.[citation needed]
Structural diversity
IgD has structural diversity throughout evolution of vertebrates because it is a structurally flexible locus to complement the function of IgM. One of the important features of IgD is that it can substitute for the function of IgM in the case of IgM defects.[7][8] B cells may express IgD by alternative RNA splicing and class switch recombination. Alternative splicing is promoted in all jawed vertebrates but class switch recombination occurs only in higher vertebrates and increases diversification of IgD.[9] In jawed fishes, the structure of the constant region is highly diverse with amplifications of Cδ exons.[10][8] Different splice variants exist due to alternative splicing. In humans and primates, IgD has three Cδ domains and a long H region with an amino-terminal region rich in alanine and threonine residues. C-terminal regions are rich in lysine, glutamate and arginine residues modified with O-glycosylation for binding a putative IgD receptor on the surface of activated T cells.[11][12] Human IgD with its H region interacts with heparin and heparan sulphate proteglycans expressed in the basophils and mast cells.[12] Mouse IgD has a shorter H region and different amino acid composition modified with N-glycosylation.
Method of coexpression
In the human heavy chain locus, 3' of the V-D-J cassette is a series of C (for constant) genes, each conferring an Ig isotype. The Cμ (IgM) gene is 3' and closest to the V-D-J cassette, with the Cδ gene appearing 3' to Cμ.
A primary mRNA transcript will contain the transcribed V-D-J cassette, and the Cμ and Cδ genes, with introns in between them.
Alternative splicing can then occur, causing a selection of either Cμ or Cδ to appear on the functional mRNA (μ mRNA and δ mRNA respectively). Alternative splicing is thought to be possible due to two polyadenylation sites, one appearing between the Cμ and Cδ, and the other 3' of Cδ (polyadenylation in the latter site would cause Cμ to be spliced away along with the intron). The precise mechanism of how the polyadenylation site is chosen remains unclear.
The resulting functional mRNA will have the V-D-J and C regions contiguous, and its translation will generate either a μ heavy chain or δ heavy chain. The heavy chains then couple with either κ or λ light chains to create the final IgM or IgD antibody.
Zinc finger protein 318 (ZNF318) has a role in the promotion of IgD expression and controlling the alternative splicing of the long pre-mRNA.[13] In immature B cells that mainly express the μ transcript, there is no ZFP318 expression, but in mature B cells with dual IgM and IgD expression, both δ and μ transcript is made and ZFP318 is expressed.[13] Enders et al. (2014)[14] found in mice that null mutations in ZFP318 resulted in no IgD expression.
Activation of immune system via IgD
Innate and adaptive Immune responses can be activated via membrane-anchored IgD that functions as a part of B-cell receptor (BCR) complexes[3] or secreted IgD that bounds to monocytes,[15] mast cells,[16] and basophil,[6][17]. Counter-intuitive to the contemporary dogmas that suggest these activated immune responses via IgD expression can potentiate autoimmune diseases and allergic inflammation, a 2010 study by Nguyen TG et al. has first demonstrated that treatments with a B-cell activating monoclonal anti-IgD antibody can attenuate disease severity in an animal model of collagen-induced arthritis.[15] This novel therapeutic effect by anti-IgD antibody treatment was later confirmed in mouse models of epidermolysis bullosa acquisita[18] and in chronic contact hypersensitivity.[19] Studies have shown that levels of secreted lgD are usually elevated in patients with an autoimmune disease, and recently it has been demonstrated that IgD enhanced the activation of peripheral blood mononuclear cells in Rheumatoid Arthritis (RA) patients leading to the hypothesis that IgD could be an immunotherapeutic target for the management of RA.[20] Activated immune responses via IgD-BCR and secreted IgD may exert suppressive effects on autoimmune diseases and allergic inflammations, suggesting a potential immune regulatory function of IgD.[21]
References
- ↑ "Metabolism of human immunoglobulin D (IgD)". J. Clin. Invest. 45 (9): 1467–78. 1966. doi:10.1172/JCI105454. PMID 5919348.
- ↑ Ohta, Yuko; Martin Flajnik (2006-07-11). "IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates". Proceedings of the National Academy of Sciences 103 (28): 10723–10728. doi:10.1073/pnas.0601407103. PMID 16818885. Bibcode: 2006PNAS..10310723O.
- ↑ 3.0 3.1 Übelhart, R; Hug, E; Bach, MP; Wossning, T; Dühren-von Minden, M; Horn, AH; Tsiantoulas, D; Kometani, K et al. (2015). "Responsiveness of B cells is regulated by the hinge region of IgD". Nat Immunol 16 (5): 534–43. doi:10.1038/ni.3141. PMID 25848865.
- ↑ "Insights into the function of IgD". Dev. Comp. Immunol. 35 (12): 1309–16. 2011. doi:10.1016/j.dci.2011.03.002. PMID 21414345.
- ↑ "Immunoglobulin D-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens". Proc. Natl. Acad. Sci. U.S.A. 90 (5): 1887–91. 1993. doi:10.1073/pnas.90.5.1887. PMID 8446604. Bibcode: 1993PNAS...90.1887N.
- ↑ 6.0 6.1 Chen, Kang; Xu, Weifeng; Wilson, Melanie; He, Bing; Miller, Norman W; Bengtén, Eva; Edholm, Eva-Stina; Santini, Paul A et al. (2009). "Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell–stimulating programs in basophils". Nature Immunology 10 (8): 889–898. doi:10.1038/ni.1748. PMID 19561614.
- ↑ Bengtén, Eva; Quiniou, Sylvie M.-A.; Stuge, Tor B.; Katagiri, Takayuki; Miller, Norman W.; Clem, L. William; Warr, Gregory W.; Wilson, Melanie (2002-09-01). "The IgH Locus of the Channel Catfish, Ictalurus punctatus, Contains Multiple Constant Region Gene Sequences: Different Genes Encode Heavy Chains of Membrane and Secreted IgD". The Journal of Immunology 169 (5): 2488–2497. doi:10.4049/jimmunol.169.5.2488. ISSN 0022-1767. PMID 12193718.
- ↑ 8.0 8.1 Preud'homme, Jean-Louis; Petit, Isabelle; Barra, Anne; Morel, Jean-Claude Lecron, Franck; Lelièvre, Eric (October 2000). "Structural and functional properties of membrane and secreted IgD". Molecular Immunology 37 (15): 871–887. doi:10.1016/s0161-5890(01)00006-2. ISSN 0161-5890. PMID 11282392.
- ↑ Ohta, Y.; Flajnik, M. (2006-07-03). "IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates". Proceedings of the National Academy of Sciences 103 (28): 10723–10728. doi:10.1073/pnas.0601407103. ISSN 0027-8424. PMID 16818885. Bibcode: 2006PNAS..10310723O.
- ↑ Chen, Kang; Cerutti, Andrea (2010-08-19). "New insights into the enigma of immunoglobulin D". Immunological Reviews 237 (1): 160–179. doi:10.1111/j.1600-065x.2010.00929.x. ISSN 0105-2896. PMID 20727035.
- ↑ Iwase, H.; Tanaka, A.; Hiki, Y.; Kokubo, T.; Ishii-Karakasa, I.; Kobayashi, Y.; Hotta, K. (1996-07-01). "Abundance of Gal 1,3GalNAc in O-Linked Oligosaccharide on Hinge Region of Polymerized IgA1 and Heat-Aggregated IgA1 from Normal Human Serum". Journal of Biochemistry 120 (1): 92–97. doi:10.1093/oxfordjournals.jbchem.a021398. ISSN 0021-924X. PMID 8864849.
- ↑ 12.0 12.1 Swenson, Christina D.; Patel, Thakor; Parekh, Raj B.; Tamma, S. M. Lakshmi; Coico, Richard F.; Thorbecke, G. Jeanette; Amin, Ashok R. (August 1998). "Human T cell IgD receptors react with O-glycans on both human IgD and IgA1". European Journal of Immunology 28 (8): 2366–2372. doi:10.1002/(sici)1521-4141(199808)28:08<2366::aid-immu2366>3.0.co;2-d. ISSN 0014-2980. PMID 9710214.
- ↑ 13.0 13.1 Murphy, K; Weaver, C (2016). Janeway's Immunobiology. New York, NY: Garland Science/Taylor and Francis. p. 195. ISBN 978-0-8153-4505-3.
- ↑ Enders, A.; Short, A.; Miosge, L.; Bergmann, H.; Sontani, Y.; Bertram, E.; Whittle, B.; Balakishnan, B. et al. (2014). "Zinc-finger protein ZFP318 is essential for expression of IgD, the alternatively spliced Igh product made by mature B lymphocytes.". Proceedings of the National Academy of Sciences 111 (12): 4513–4518. doi:10.1073/pnas.1402739111. PMID 24616512. Bibcode: 2014PNAS..111.4513E.
- ↑ 15.0 15.1 Nguyen, Tue G.; Little, Christopher B.; Yenson, Vanessa M.; Jackson, Christopher J.; McCracken, Sharon A.; Warning, Julia; Stevens, Veronica; Gallery, Eileen G. et al. (2010). "Anti-IgD antibody attenuates collagen-induced arthritis by selectively depleting mature B-cells and promoting immune tolerance". Journal of Autoimmunity 35 (1): 86–97. doi:10.1016/j.jaut.2010.03.003. ISSN 0896-8411. PMID 20456921.
- ↑ Zhai, Guan-Ting; Wang, Hai; Li, Jing-Xian; Cao, Ping-Ping; Jiang, Wen-Xiu; Song, Jia; Yao, Yin; Wang, Zhi-Chao et al. (2018). "IgD-activated mast cells induce IgE synthesis in B cells in nasal polyps". Journal of Allergy and Clinical Immunology 142 (5): 1489–1499.e23. doi:10.1016/j.jaci.2018.07.025. ISSN 0091-6749. PMID 30102935.
- ↑ Shan, Meimei; Carrillo, Jorge; Yeste, Ada; Gutzeit, Cindy; Segura-Garzón, Daniel; Walland, A. Cooper; Pybus, Marc; Grasset, Emilie K. et al. (2018). "Secreted IgD Amplifies Humoral T Helper 2 Cell Responses by Binding Basophils via Galectin-9 and CD44". Immunity 49 (4): 709–724.e8. doi:10.1016/j.immuni.2018.08.013. ISSN 1074-7613. PMID 30291028.
- ↑ Kulkarni, Upasana; Karsten, Christian M.; Kohler, Thomas; Hammerschmidt, Sven; Bommert, Kurt; Tiburzy, Benjamin; Meng, Lingzhang; Thieme, Lara et al. (2016). "IL-10 mediates plasmacytosis-associated immunodeficiency by inhibiting complement-mediated neutrophil migration". Journal of Allergy and Clinical Immunology 137 (5): 1487–1497.e6. doi:10.1016/j.jaci.2015.10.018. ISSN 0091-6749. PMID 26653800.
- ↑ Nguyen, Tue G. (2018). "Immune-modulation via IgD B-cell receptor suppresses allergic skin inflammation in experimental contact hypersensitivity models despite a Th2-favoured humoral response". Immunology Letters 203: 29–39. doi:10.1016/j.imlet.2018.09.008. ISSN 0165-2478. PMID 30218740.
- ↑ Wu, Yujing (2016). "The Elevated Secreted Immunoglobulin D Enhanced the Activation of Peripheral Blood Mononuclear Cells in Rheumatoid Arthritis". PLOS ONE 24 (1): 544–551. doi:10.1080/16078454.2019.1642553. PMID 31315540.
- ↑ Nguyen, Tue Gia (2021-01-07). "The therapeutic implications of activated immune responses via the enigmatic immunoglobulin D" (in en). International Reviews of Immunology 41 (2): 107–122. doi:10.1080/08830185.2020.1861265. ISSN 0883-0185. PMID 33410368. https://www.tandfonline.com/doi/full/10.1080/08830185.2020.1861265.
External links
- Immunoglobulin+D at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

