Biology:Anion exchanger family
| Anion Exchanger, bicarbonate transporter family | |
|---|---|
| Identifiers | |
| Symbol | HCO3_cotransp |
| InterPro | IPR003020 |
| PROSITE | PDOC00192 |
| TCDB | 2.A.31 |
The anion exchanger family (TC# 2.A.31, also named bicarbonate transporter family) is a member of the large APC superfamily of secondary carriers.[1] Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria (e.g., in Enterococcus faecium, 372 aas; gi 22992757; 29% identity in 90 residues). Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.
Family overview
| Bicarbonate transporter, C-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 a low energy structure for the final cytoplasmic loop of band 3, nmr, minimized average structure | |||||||||
| Identifiers | |||||||||
| Symbol | HCO3_transpt_C | ||||||||
| Pfam | PF00955 | ||||||||
| Pfam clan | CL0062 | ||||||||
| InterPro | IPR011531 | ||||||||
| PROSITE | PDOC00192 | ||||||||
| SCOP2 | 1btr / SCOPe / SUPFAM | ||||||||
| |||||||||
| Band 3 cytoplasmic domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
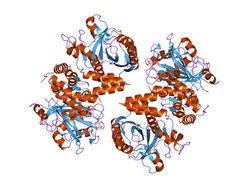 crystal structure of the cytoplasmic domain of human erythrocyte band-3 protein | |||||||||
| Identifiers | |||||||||
| Symbol | Band_3_cyto | ||||||||
| Pfam | PF07565 | ||||||||
| Pfam clan | CL0340 | ||||||||
| InterPro | IPR013769 | ||||||||
| SCOP2 | 1hyn / SCOPe / SUPFAM | ||||||||
| TCDB | 2.A.31 | ||||||||
| OPM superfamily | 284 | ||||||||
| OPM protein | 1btq | ||||||||
| |||||||||
Bicarbonate (HCO3 −) transport mechanisms are the principal regulators of pH in animal cells. Such transport also plays a vital role in acid-base movements in the stomach, pancreas, intestine, kidney, reproductive organs and the central nervous system. Functional studies have suggested different HCO3 − transport modes.
- Anion exchanger proteins exchange HCO3 − for Cl− in a reversible, electroneutral manner.[2]
- Na+/HCO3 − co-transport proteins mediate the coupled movement of Na+ and HCO3 − across plasma membranes, often in an electrogenic manner.[3]
Sequence analysis of the two families of HCO3 − transporters that have been cloned to date (the anion exchangers and Na+/HCO3 − co-transporters) reveals that they are homologous. This is not entirely unexpected, given that they both transport HCO3 − and are inhibited by a class of pharmacological agents called disulphonic stilbenes.[4] They share around ~25-30% sequence identity, which is distributed along their entire sequence length, and have similar predicted membrane topologies, suggesting they have ~10 transmembrane (TM) domains.
A conserved domain is found at the C terminus of many bicarbonate transport proteins. It is also found in some plant proteins responsible for boron transport.[5] In these proteins it covers almost the entire length of the sequence.
The Band 3 anion exchange proteins that exchange bicarbonate are the most abundant polypeptide in the red blood cell membrane, comprising 25% of the total membrane protein. The cytoplasmic domain of band 3 functions primarily as an anchoring site for other membrane-associated proteins. Included among the protein ligands of this domain are ankyrin, protein 4.2, protein 4.1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphofructokinase, aldolase, hemoglobin, hemichromes, and the protein tyrosine kinase (p72syk).[6]
Anion exchangers in humans
In humans, anion exchangers fall under the solute carrier family 4 (SLC4) family, which is composed of 10 paralogous members (SLC4A1-5; SLC4A7-11). Nine encode proteins that transport HCO−3. Functionally, eight of these proteins fall into two major groups: three Cl-HCO−3 exchangers (AE1-3) and five Na+-coupled HCO−3 transporters (NBCe1, NBCe2, NBCn1, NBCn2, NDCBE). Two of the Na+-coupled transporters (NBCe1, NBCe2) are electrogenic; the other three Na+-coupled HCO−3 transporters and all three AEs are electroneutral.[7][8] Two others (AE4, SLC4A9 and BTR1, SLC4A11) are not characterized. Most, though not all, are inhibited by 4,4'-diisothiocyanatostilbene-2,2'-disulfonate (DIDS). SLC4 proteins play roles in acid-base homeostasis, transport of H+ or HCO−3 by epithelia (e.g. absorption of HCO−3 in the renal proximal tubule, secretion of HCO−3 in the pancreatic duct), as well as the regulation of cell volume and intracellular pH.[8]
Based on their hydropathy plots all SLC4 proteins are hypothesized to share a similar topology in the cell membrane. They have relatively long cytoplasmic N-terminal domains composed of a few hundred to several hundred residues, followed by 10-14 transmembrane (TM) domains, and end with relatively short cytoplasmic C-terminal domains composed of ~30 to ~90 residues. Although the C-terminal domain comprises a small percentage of the size of the protein, this domain in some cases, has (i) binding motifs that may be important for protein-protein interactions (e.g., AE1, AE2, and NBCn1), (ii) is important for trafficking to the cell membrane (e.g., AE1 and NBCe1), and (iii) may provide sites for regulation of transporter function via protein kinase A phosphorylation (e.g., NBCe1).[9]
The SLC4 family comprises the following proteins.
Anion exchanger 1
The human anion exchanger 1 (AE1 or Band 3) binds carbonic anhydrase II (CAII) forming a "transport metabolon" as CAII binding activates AE1 transport activity about 10 fold.[10] AE1 is also activated by interaction with glycophorin, which also functions to target it to the plasma membrane.[11] The membrane-embedded C-terminal domains may each span the membrane 13-16 times. According to the model of Zhu et al. (2003), AE1 in humans spans the membrane 16 times, 13 times as α-helix, and three times (TMSs 10, 11 and 14) possibly as β-strands.[12] AE1 preferentially catalyzes anion exchange (antiport) reactions. Specific point mutations in human anion exchanger 1 (AE1) convert this electroneutral anion exchanger into a monovalent cation conductance. The same transport site within the AE1 spanning domain is involved in both anion exchange and cation transport.[13]
AE1 in human red blood cells has been shown to transport a variety of inorganic and organic anions. Divalent anions may be symported with H+. Additionally, it catalyzes flipping of several anionic amphipathic molecules such as sodium dodecyl sulfate (SDS) and phosphatidic acid from one monolayer of the phospholipid bilayer to the other monolayer. The rate of flipping is sufficiently rapid to suggest that this AE1-catalyzed process is physiologically important in red blood cells and possibly in other animal tissues as well. Anionic phospholipids and fatty acids are likely to be natural substrates. However, the mere presence of TMSs enhances the rates of lipid flip-flop.[14][15]
Structure
The crystal structure of AE1 (CTD) at 3.5 angstroms has been determined.[16] The structure is locked in an outward-facing open conformation by an inhibitor. Comparing this structure with a substrate-bound structure of the uracil transporter UraA in an inward-facing conformation allowed identification of the likely anion-binding position in the AE1 (CTD), and led to proposal of a possible transport mechanism that could explain why selected mutations lead to disease. The 3-D structure confirmed that the AE family is a member of the APC superfamily.[9]
There are several crystal structures available for the AE1 protein in RCSB (links are also available in TCDB).
- AE1: 1BH7, 1BNX, 1BTQ, 1BTR, 1BTS, 1BTT, 1BZK, 2BTA, 1HYN, 2BTB, 3BTB, 2BTA, 2BTB, 3BTB, 4KY9, PDB: 4YZF, 1HYN, 5A16
Other members
Renal Na+:HCO−3 cotransporters have been found to be members of the AE family. They catalyze the reabsorption of HCO−3 in the renal proximal tubule in an electrogenic process that is inhibited by typical stilbene inhibitors of AE such as DIDS and SITS. They are also found in many other body tissues. At least two genes encode these symporters in any one mammal. A 10 TMS model has been presented,[17] but this model conflicts with the 14 TMS model proposed for AE1. The transmembrane topology of the human pancreatic electrogenic Na+:HO−3 transporter, NBC1, has been studied.[18] A TMS topology with N- and C-termini in the cytoplasm has been suggested. An extracellular loop determines the stoichiometry of Na+-HCO−3 cotransporters.[19]
In addition to the Na+-independent anion exchangers (AE1-3) and the Na+:HCO−3 cotransporters (NBCs) (which may be either electroneutral or electrogenic), a Na+-driven HCO−3/Cl− exchanger (NCBE) has been sequenced and characterized.[20] It transports Na+ + HCO−3 preferentially in the inward direction and H+ + Cl− in the outward direction. This NCBE is widespread in mammalian tissues where it plays an important role in cytoplasmic alkalinization. For example, in pancreatic β-cells, it mediates a glucose-dependent rise in pH related to insulin secretion.
Animal cells in tissue culture expressing the gene-encoding the ABC-type chloride channel protein CFTR (TC# 3.A.1.202.1) in the plasma membrane have been reported to exhibit cyclic AMP-dependent stimulation of AE activity. Regulation was independent of the Cl− conductance function of CFTR, and mutations in the nucleotide-binding domain #2 of CFTR altered regulation independently of their effects on chloride channel activity. These observations may explain impaired HCO−3 secretion in cystic fibrosis patients.
Anion exchangers in plants and fungi
Plants and yeast have anion transporters that in both the pericycle cells of plants and the plasma membrane of yeast cells export borate or boric acid (pKa = 9.2).[5] In A. thaliana, boron is exported from pericycle cells into the root stellar apoplasm against a concentration gradient for uptake into the shoots. In S. cerevisiae, export is also against a concentration gradient. The yeast transporter recognizes HCO−3, I−, Br−, NO−3 and Cl−, which may be substrates. Tolerance to boron toxicity in cereals is known to be associated with reduced tissue accumulation of boron. Expression of genes from roots of boron-tolerant wheat and barley with high similarity to efflux transporters from Arabidopsis and rice lowered boron concentrations due to an efflux mechanism.[21] The mechanism of energy coupling is not known, nor is it known if borate or boric acid is the substrate. Several possibilities (uniport, anion:anion exchange and anion:cation exchange) can account for the data.[5]
Transport reactions
The physiologically relevant transport reaction catalyzed by anion exchangers of the AE family is:[9]
- Cl− (in) + HCO−3 (out) ⇌ Cl− (out) + HCO−3 (in).
That for the Na+:HCO3- cotransporters is:
- Na+ (out) + nHCO−3 (out) → Na+ (in) + nHCO−3 (in).
That for the Na+/HCO−3:H+/Cl− exchanger is:
- Na+ (out) + HCO−3 (out) + H+ (in) + Cl− (in) ⇌ Na+ (in) + HCO−3 (in) + H+ (out) + Cl− (out).
That for the boron efflux protein of plants and yeast is:
- Boron (in) → Boron (out)
See also
References
- ↑ "Expansion of the APC superfamily of secondary carriers". Proteins 82 (10): 2797–811. October 2014. doi:10.1002/prot.24643. PMID 25043943.
- ↑ "Molecular biology of the anion exchanger gene family". International Review of Cytology 123: 177–99. 1990. doi:10.1016/S0074-7696(08)60674-9. ISBN 9780123645234. PMID 2289848.
- ↑ "The electrogenic Na/HCO3 cotransporter". Wiener Klinische Wochenschrift 109 (12–13): 445–56. June 1997. PMID 9261985.
- ↑ "Cloning and functional expression of a human kidney Na+:HCO3- cotransporter". The Journal of Biological Chemistry 272 (31): 19111–4. August 1997. doi:10.1074/jbc.272.31.19111. PMID 9235899.
- ↑ 5.0 5.1 5.2 "Arabidopsis boron transporter for xylem loading". Nature 420 (6913): 337–40. November 2002. doi:10.1038/nature01139. PMID 12447444. Bibcode: 2002Natur.420..337T.
- ↑ "Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3". Blood 96 (9): 2925–33. November 2000. doi:10.1182/blood.V96.9.2925. PMID 11049968.
- ↑ "Cloning and characterization of an electrogenic Na/HCO3- cotransporter from the squid giant fiber lobe". American Journal of Physiology. Cell Physiology 292 (6): C2032-45. June 2007. doi:10.1152/ajpcell.00544.2006. PMID 17267543.
- ↑ 8.0 8.1 "The SLC4 family of bicarbonate (HCO3−) transporters". Molecular Aspects of Medicine 34 (2–3): 159–82. 2013-06-01. doi:10.1016/j.mam.2012.10.008. PMID 23506864.
- ↑ 9.0 9.1 9.2 Saier, MH Jr.. "2.A.31 The Anion Exchanger (AE) Family". Saier Lab Bioinformatics Group @ UCSD / SDSC. http://www.tcdb.org/search/result.php?tc=2.A.31.
- ↑ "A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers". The Journal of Biological Chemistry 276 (51): 47886–94. December 2001. doi:10.1074/jbc.M105959200. PMID 11606574.
- ↑ "Distinct regions of human glycophorin A enhance human red cell anion exchanger (band 3; AE1) transport function and surface trafficking". The Journal of Biological Chemistry 278 (35): 32954–61. August 2003. doi:10.1074/jbc.M302527200. PMID 12813056.
- ↑ "Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1". The Journal of Biological Chemistry 278 (5): 3112–20. January 2003. doi:10.1074/jbc.M207797200. PMID 12446737.
- ↑ "Dual transport properties of anion exchanger 1: the same transmembrane segment is involved in anion exchange and in a cation leak". The Journal of Biological Chemistry 286 (11): 8909–16. March 2011. doi:10.1074/jbc.M110.166819. PMID 21257764.
- ↑ "Membrane-spanning peptides induce phospholipid flop: a model for phospholipid translocation across the inner membrane of E. coli". Biochemistry 40 (35): 10500–6. September 2001. doi:10.1021/bi010627+. PMID 11523991.
- ↑ "Molecular simulations of lipid flip-flop in the presence of model transmembrane helices". Biochemistry 49 (35): 7665–73. September 2010. doi:10.1021/bi100878q. PMID 20666375.
- ↑ "Crystal structure of the anion exchanger domain of human erythrocyte band 3". Science 350 (6261): 680–4. November 2015. doi:10.1126/science.aaa4335. PMID 26542571. Bibcode: 2015Sci...350..680A. http://wrap.warwick.ac.uk/74238/1/WRAP_0380721-lf-131115-science_cameron.pdf.
- ↑ "Electrogenic Na+/HCO3- cotransporters: cloning and physiology". Annual Review of Physiology 61: 699–723. 1999-01-01. doi:10.1146/annurev.physiol.61.1.699. PMID 10099707.
- ↑ "Identification of membrane topography of the electrogenic sodium bicarbonate cotransporter pNBC1 by in vitro transcription/translation". Biochemistry 42 (3): 755–65. January 2003. doi:10.1021/bi026826q. PMID 12534288.
- ↑ "Role of an extracellular loop in determining the stoichiometry of Na+-HCO3− cotransporters". The Journal of Physiology 589 (Pt 4): 877–90. February 2011. doi:10.1113/jphysiol.2010.198713. PMID 21224233.
- ↑ "The Na+-driven Cl-/HCO3- exchanger. Cloning, tissue distribution, and functional characterization". The Journal of Biological Chemistry 275 (45): 35486–90. November 2000. doi:10.1074/jbc.C000456200. PMID 10993873.
- ↑ "Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley". Plant & Cell Physiology 48 (12): 1673–8. December 2007. doi:10.1093/pcp/pcm159. PMID 18003669.
 |

