Biology:Hyaline cartilage
| Hyaline cartilage | |
|---|---|
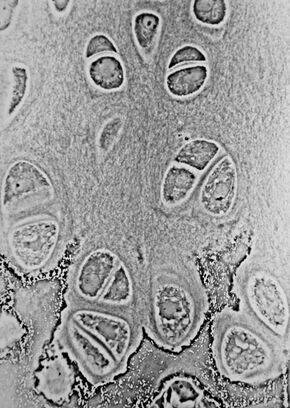 Light micrograph of undecalcified hyaline cartilage showing microanatomy of chondrocytes and organelles, lacunae and matrix. | |
| Anatomical terminology |
Hyaline cartilage is the glass-like (hyaline) and translucent cartilage found on many joint surfaces. It is also most commonly found in the ribs, nose, larynx, and trachea.[1] Hyaline cartilage is pearl-gray in color, with a firm consistency and has a considerable amount of collagen. It contains no nerves or blood vessels, and its structure is relatively simple.
Structure
Hyaline cartilage is covered externally by a fibrous membrane known as the perichondrium or, when it's along articulating surfaces, the synovial membrane. This membrane contains vessels that provide the cartilage with nutrition through diffusion.
Hyaline cartilage matrix is primarily made of type II collagen and chondroitin sulphate, both of which are also found in elastic cartilage.
Hyaline cartilage exists on the sternal ends of the ribs, in the larynx, trachea, and bronchi, and on the articulating surfaces of bones. It gives the structures a definite but pliable form. The presence of collagen fibres makes such structures and joints strong, but with limited mobility and flexibility.
Hyaline cartilage is the most prevalent type of cartilage. It also forms the temporary embryonic skeleton, which is gradually replaced by bone, and the skeleton of elasmobranch fish.
Microanatomy
When a slice of hyaline cartilage is examined under the microscope, it is shown to consist of cells (chondrocytes) of a rounded or bluntly angular form, lying in groups of two or more in a granular, or almost homogeneous matrix. When arranged in groups of two or more, the chondrocytes have rounded, but generally straight outlines; where they are in contact with each other, and in the rest of their circumference, they are rounded.
They consist of translucent protoplasm with fine interlacing filaments and minute granules are sometimes present. Embedded in this are one or two round nuclei, having the usual intranuclear network.
The cells are contained in cavities in the matrix, called cartilage lacunae. These cavities are actually artificial gaps formed from the shrinking of the cells during the staining and setting of the tissue for examination. The inter-territorial space between the isogenous cell groups contains relatively more collagen fibres, allowing it to maintain its shape while the actual cells shrink, creating the lacunae. This constitutes the so-called 'capsule' of the space. Each lacuna is usually occupied by a single cell, but during mitosis, it may contain two, four, or even eight cells.
Articular cartilage

Articular cartilage is hyaline cartilage on the articular surfaces of bones,[3] and lies inside the joint cavity of synovial joints, bathed in synovial fluid produced by the synovial membrane, which lines the walls of the cavity.
Though it is often found in close contact with menisci and articular disks, articular cartilage is not considered a part of either of these structures, which are made entirely of fibrocartilage.
The articular cartilage extracellular matrix (ECM) has a highly specialized architecture that is zonally organized: the superficial zone consists mostly of collagen II fibers aligned parallel to the articular surface to resist shear forces, whereas the deep zone consists of the same fibers aligned perpendicularly to the bone interface to absorb compressive loads.[2]
The biochemical breakdown of the articular cartilage results in osteoarthritis – the most common type of joint disease.[4] Osteoarthritis affects over 30 million individuals in the United States alone, and is the leading cause of chronic disability amongst the elderly.[5]
Articular cartilage development begins with interzone condensation of a Collagen II positive limb bud at the future joint site. This is followed by definition of specific cellular subtypes (meniscal progenitors, articular progenitors, synovial progenitors, and ligament progenitors) that will eventually form the joint capsule. Finally, the joint capsule matures and forms a cavity, with a central meniscus, and an encasement of synovium.[6] This final structure will form several distinct layers of the articular cartilage found in all synovial joints including the Deep Zone (closest to the bone), Middle Zone, and Superficial Zone (closest to the synovial fluid).
Maintenance of articular cartilage is guided by a balance of anabolic (cartilage generating)[7][8] and catabolic (cartilage degrading factors),[9][10] in a manner similar to the maintenance of bone.[11] Over the lifetime of the organism, anabolic factors and catabolic factors are generally in balance, however, as the organism ages, catabolism predominates and cartilage begins to degrade. Eventually, the loss of hyaline cartilage matrix and reduction in the chondrocyte content of the hyaline cartilage matrix results in the development of joint disease such as Osteoarthritis (OA). Overexpression of hyaline-cartilage specific anabolic factors, such as FGF18, or appears to restore the balance between cartilage loss and generation.[12][13]
Additional images
See also
- Articular cartilage damage
- Articular cartilage injuries
- Articular cartilage repair
- List of distinct cell types in the adult human body
References
- ↑ Adele, Knibbs (2003). "The Leeds Histology Guide" (in en). https://www.histology.leeds.ac.uk/bone/cartilage_types.php.
- ↑ 2.0 2.1 Zhao, Feng; Bautista, Catherine A.; Park, Hee Jun; Mazur, Courtney M.; Aaron, Roy K.; Bilgen, Bahar (2016). "Effects of Chondroitinase ABC-Mediated Proteoglycan Digestion on Decellularization and Recellularization of Articular Cartilage". PLOS ONE 11 (7): e0158976. doi:10.1371/journal.pone.0158976. ISSN 1932-6203. PMID 27391810. Bibcode: 2016PLoSO..1158976B.
-"The work is made available under the Creative Commons CC0 public domain dedication." - ↑ "Wheeless' Textbook of Orthopaedics". 22 July 2020. http://www.wheelessonline.com/ortho/articular_cartilage.
- ↑ Brown, Angelina. "Coping with Osteoarthritis". https://medium.com/@brownangelina71/coping-with-osteoarthritis-c8d707afab6a.
- ↑ "Osteoarthritis Fact Sheet". Center for Disease Control and Prevention. https://www.cdc.gov/arthritis/basics/osteoarthritis.htm.
- ↑ Wilkinson, J. Mark; Zeggini, Eleftheria (2021-09-01). "The Genetic Epidemiology of Joint Shape and the Development of Osteoarthritis" (in en). Calcified Tissue International 109 (3): 257–276. doi:10.1007/s00223-020-00702-6. ISSN 1432-0827. PMID 32393986. PMC 8403114. https://doi.org/10.1007/s00223-020-00702-6.
- ↑ Davidson, David; Blanc, Antoine; Filion, Dominic; Wang, Huifen; Plut, Paul; Pfeffer, Gerald; Buschmann, Michael D.; Henderson, Janet E. (2005-05-27). "Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis". The Journal of Biological Chemistry 280 (21): 20509–20515. doi:10.1074/jbc.M410148200. ISSN 0021-9258. PMID 15781473.
- ↑ Takahata, Yoshifumi; Hagino, Hiromasa; Kimura, Ayaka; Urushizaki, Mitsuki; Yamamoto, Shiori; Wakamori, Kanta; Murakami, Tomohiko; Hata, Kenji et al. (2022-04-23). "Regulatory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis". International Journal of Molecular Sciences 23 (9): 4672. doi:10.3390/ijms23094672. ISSN 1422-0067. PMID 35563063.
- ↑ Alvaro-Gracia, J. M. (February 2004). "Licofelone--clinical update on a novel LOX/COX inhibitor for the treatment of osteoarthritis". Rheumatology 43 (Suppl 1): i21–25. doi:10.1093/rheumatology/keh105. ISSN 1462-0324. PMID 14752172. https://pubmed.ncbi.nlm.nih.gov/14752172/.
- ↑ Li, Ting; Peng, Jie; Li, Qingqing; Shu, Yuan; Zhu, Peijun; Hao, Liang (2022-07-08). "The Mechanism and Role of ADAMTS Protein Family in Osteoarthritis". Biomolecules 12 (7): 959. doi:10.3390/biom12070959. ISSN 2218-273X. PMID 35883515.
- ↑ Maeda, Kazuhiro; Kobayashi, Yasuhiro; Koide, Masanori; Uehara, Shunsuke; Okamoto, Masanori; Ishihara, Akihiro; Kayama, Tomohiro; Saito, Mitsuru et al. (2019-11-06). "The Regulation of Bone Metabolism and Disorders by Wnt Signaling". International Journal of Molecular Sciences 20 (22): 5525. doi:10.3390/ijms20225525. ISSN 1422-0067. PMID 31698687.
- ↑ Hollander, Judith M.; Goraltchouk, Alex; Rawal, Miraj; Liu, Jingshu; Luppino, Francesco; Zeng, Li; Seregin, Alexey (2023-03-06). "Adeno-Associated Virus-Delivered Fibroblast Growth Factor 18 Gene Therapy Promotes Cartilage Anabolism". Cartilage: 19476035231158774. doi:10.1177/19476035231158774. ISSN 1947-6043. PMID 36879540. PMC 10807742. https://pubmed.ncbi.nlm.nih.gov/36879540/.
- ↑ Moore, E. E.; Bendele, A. M.; Thompson, D. L.; Littau, A.; Waggie, K. S.; Reardon, B.; Ellsworth, J. L. (July 2005). "Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis". Osteoarthritis and Cartilage 13 (7): 623–631. doi:10.1016/j.joca.2005.03.003. ISSN 1063-4584. PMID 15896984. https://pubmed.ncbi.nlm.nih.gov/15896984/.
External links
- Histology image: 03301lba – Histology Learning System at Boston University
- UIUC Histology Subject 331
 |



