Biology:MNase-seq
MNase-seq, short for micrococcal nuclease digestion with deep sequencing,[1][2][3][4] is a molecular biological technique that was first pioneered in 2006 to measure nucleosome occupancy in the C. elegans genome,[1] and was subsequently applied to the human genome in 2008.[2] Though, the term ‘MNase-seq’ had not been coined until a year later, in 2009.[3] Briefly, this technique relies on the use of the non-specific endo-exonuclease micrococcal nuclease, an enzyme derived from the bacteria Staphylococcus aureus, to bind and cleave protein-unbound regions of DNA on chromatin. DNA bound to histones or other chromatin-bound proteins (e.g. transcription factors) may remain undigested. The uncut DNA is then purified from the proteins and sequenced through one or more of the various Next-Generation sequencing methods.[5]
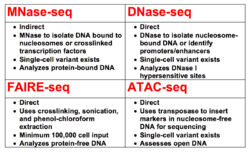
MNase-seq is one of four classes of methods used for assessing the status of the epigenome through analysis of chromatin accessibility. The other three techniques are DNase-seq, FAIRE-seq, and ATAC-seq.[4] While MNase-seq is primarily used to sequence regions of DNA bound by histones or other chromatin-bound proteins,[2] the other three are commonly used for: mapping Deoxyribonuclease I hypersensitive sites (DHSs),[6] sequencing the DNA unbound by chromatin proteins,[7] or sequencing regions of loosely packaged chromatin through transposition of markers,[8][9] respectively.[4]
History
Micrococcal nuclease (MNase) was first discovered in S. aureus in 1956,[10] protein crystallized in 1966,[11] and characterized in 1967.[12] MNase digestion of chromatin was key to early studies of chromatin structure; being used to determine that each nucleosomal unit of chromatin was composed of approximately 200bp of DNA.[13] This, alongside Olins’ and Olins’ “beads on a string” model,[14] confirmed Kornberg’s ideas regarding the basic chromatin structure.[15] Upon additional studies, it was found that MNase could not degrade histone-bound DNA shorter than ~140bp and that DNase I and II could degrade the bound DNA to as low as 10bp.[16][17] This ultimately elucidated that ~146bp of DNA wrap around the nucleosome core,[18] ~50bp linker DNA connect each nucleosome,[19] and that 10 continuous base-pairs of DNA tightly bind to the core of the nucleosome in intervals.[17]
In addition to being used to study chromatin structure, micrococcal nuclease digestion had been used in oligonucleotide sequencing experiments since its characterization in 1967.[20] MNase digestion was additionally used in several studies to analyze chromatin-free sequences, such as yeast (Saccharomyces cerevisiae) mitochondrial DNA[21] as well as bacteriophage DNA[22][23] through its preferential digestion of adenine and thymine-rich regions.[24] In the early 1980s, MNase digestion was used to determine the nucleosomal phasing and associated DNA for chromosomes from mature SV40,[25] fruit flies (Drosophila melanogaster),[26] yeast,[27] and monkeys,[28] among others. The first study to use this digestion to study the relevance of chromatin accessibility to gene expression in humans was in 1985. In this study, nuclease was used to find the association of certain oncogenic sequences with chromatin and nuclear proteins.[29] Studies utilizing MNase digestion to determine nucleosome positioning without sequencing or array information continued into the early 2000s.[30]
With the advent of whole genome sequencing in the late 1990s and early 2000s, it became possible to compare purified DNA sequences to the eukaryotic genomes of S. cerevisiae,[31] Caenorhabditis elegans,[32] D. melanogaster,[33] Arabidopsis thaliana,[34] Mus musculus,[35] and Homo sapiens.[36] MNase digestion was first applied to genome-wide nucleosome occupancy studies in S. cerevisiae[37] accompanied by analyses through microarrays to determine which DNA regions were enriched with MNase-resistant nucleosomes. MNase-based microarray analyses were often utilized at genome-wide scales for yeast[38][39] and in limited genomic regions in humans[40][41] to determine nucleosome positioning, which could be used as an inference for transcriptional inactivation.
In 2006, Next-Generation sequencing was first coupled with MNase digestion to explore nucleosome positioning and DNA sequence preferences in C. elegans,.[1] This was the first example of MNase-seq in any organism.
It was not until 2008, around the time Next-Generation sequencing was becoming more widely available, when MNase digestion was combined with high-throughput sequencing, namely Solexa/Illumina sequencing, to study nucleosomal positioning at a genome-wide scale in humans.[2] A year later, the terms “MNase-Seq” and “MNase-ChIP”, for micrococcal nuclease digestion with chromatin immunoprecipitation, were finally coined.[3] Since its initial application in 2006,[1] MNase-seq has been utilized to deep sequence DNA associated with nucleosome occupancy and epigenomics across eukaryotes.[5] As of February 2020, MNase-seq is still applied to assay accessibility in chromatin.[42]
Description
Chromatin is dynamic and the positioning of nucleosomes on DNA changes through the activity of various transcription factors and remodeling complexes, approximately reflecting transcriptional activity at these sites. DNA wrapped around nucleosomes are generally inaccessible to transcription factors.[43] Hence, MNase-seq can be used to indirectly determine which regions of DNA are transcriptionally inaccessible by directly determining which regions are bound to nucleosomes.[5]
In a typical MNase-seq experiment, eukaryotic cell nuclei are first isolated from a tissue of interest. Then, MNase-seq uses the endo-exonuclease micrococcal nuclease to bind and cleave protein-unbound regions of DNA of eukaryotic chromatin, first cleaving and resecting one strand, then cleaving the antiparallel strand as well.[3] The chromatin can be optionally crosslinked with formaldehyde.[44] MNase requires Ca2+ as a cofactor, typically with a final concentration of 1mM.[5][12] If a region of DNA is bound by the nucleosome core (i.e. histones) or other chromatin-bound proteins (e.g. transcription factors), then MNase is unable to bind and cleave the DNA. Nucleosomes or the DNA-protein complexes can be purified from the sample and the bound DNA can be subsequently purified via gel electrophoresis and extraction. The purified DNA is typically ~150bp, if purified from nucleosomes,[2] or shorter, if from another protein (e.g. transcription factors).[45] This makes short-read, high-throughput sequencing ideal for MNase-seq as reads for these technologies are highly accurate but can only cover a couple hundred continuous base-pairs in length.[46] Once sequenced, the reads can be aligned to a reference genome to determine which DNA regions are bound by nucleosomes or proteins of interest, with tools such as Bowtie.[4] The positioning of nucleosomes elucidated, through MNase-seq, can then be used to predict genomic expression[47] and regulation[48] at the time of digestion.
Extended Techniques
MNase-ChIP/CUT&RUN sequencing
Recently, MNase-seq has also been implemented in determining where transcription factors bind on the DNA.[49][50] Classical ChIP-seq displays issues with resolution quality, stringency in experimental protocol, and DNA fragmentation.[50] Classical ChIP-seq typically uses sonication to fragment chromatin, which biases heterochromatic regions due to the condensed and tight binding of chromatin regions to each other.[50] Unlike histones, transcription factors only transiently bind DNA. Other methods, such as sonication in ChIP-seq, requiring the use of increased temperatures and detergents, can lead to the loss of the factor. CUT&RUN sequencing is a novel form of an MNase-based immunoprecipitation. Briefly, it uses an MNase tagged with an antibody to specifically bind DNA-bound proteins that present the epitope recognized by that antibody. Digestion then specifically occurs at regions surrounding that transcription factor, allowing for this complex to diffuse out of the nucleus and be obtained without having to worry about significant background nor the complications of sonication. The use of this technique does not require high temperatures or high concentrations of detergent. Furthermore, MNase improves chromatin digestion due to its exonuclease and endonuclease activity. Cells are lysed in an SDS/Triton X-100 solution. Then, the MNase-antibody complex is added. And finally, the protein-DNA complex can be isolated, with the DNA being subsequently purified and sequenced. The resulting soluble extract contains a 25-fold enrichment in fragments under 50bp. This increased enrichment results in cost-effective high-resolution data.[50]
Single-cell MNase-seq
Single-cell micrococcal nuclease sequencing (scMNase-seq) is a novel technique that is used to analyze nucleosome positioning and to infer chromatin accessibility with the use of only a single-cell input.[51] First, cells are sorted into single aliquots using fluorescence-activated cell sorting (FACS).[51] The cells are then lysed and digested with micrococcal nuclease. The isolated DNA is subjected to PCR amplification and then the desired sequence is isolated and analyzed.[51] The use of MNase in single-cell assays results in increased detection of regions such as DNase I hypersensitive sites as well as transcription factor binding sites.[51]
Comparison to other Chromatin Accessibility Assays
MNase-seq is one of four major methods (DNase-seq, MNase-seq, FAIRE-seq, and ATAC-seq) for more direct determination of chromatin accessibility and the subsequent consequences for gene expression.[52] All four techniques are contrasted with ChIP-seq, which relies on the inference that certain marks on histone tails are indicative of gene activation or repression,[53] not directly assessing nucleosome positioning, but instead being valuable for the assessment of histone modifier enzymatic function.[4]
DNase-seq
As with MNase-seq,[2] DNase-seq was developed by combining an existing DNA endonuclease[6] with Next-Generation sequencing technology to assay chromatin accessibility.[54] Both techniques have been used across several eukaryotes to ascertain information on nucleosome positioning in the respective organisms[4] and both rely on the same principle of digesting open DNA to isolate ~140bp bands of DNA from nucleosomes[2][55] or shorter bands if ascertaining transcription factor information.[45][55] Both techniques have recently been optimized for single-cell sequencing, which corrects for one of the major disadvantages of both techniques; that being the requirement for high cell input.[56][51]
At sufficient concentrations, DNase I is capable of digesting nucleosome-bound DNA to 10bp, whereas micrococcal nuclease cannot.[17] Additionally, DNase-seq is used to identify DHSs, which are regions of DNA that are hypersensitive to DNase treatment and are often indicative of regulatory regions (e.g. promoters or enhancers).[57] An equivalent effect is not found with MNase. As a result of this distinction, DNase-seq is primarily utilized to directly identify regulatory regions, whereas MNase-seq is used to identify transcription factor and nucleosomal occupancy to indirectly infer effects on gene expression.[4]
FAIRE-seq
FAIRE-seq differs more from MNase-seq than does DNase-seq.[4] FAIRE-seq was developed in 2007[7] and combined with Next-Generation sequencing three years later to study DHSs.[58] FAIRE-seq relies on the use of formaldehyde to crosslink target proteins with DNA and then subsequent sonication and phenol-chloroform extraction to separate non-crosslinked DNA and crosslinked DNA. The non-crosslinked DNA is sequenced and analyzed, allowing for direct observation of open chromatin.[59]
MNase-seq does not measure chromatin accessibility as directly as FAIRE-seq. However, unlike FAIRE-seq, it does not necessarily require crosslinking,[5] nor does it rely on sonication,[4] but it may require phenol and chloroform extraction.[5] Two major disadvantages of FAIRE-seq, relative to the other three classes, are the minimum required input of 100,000 cells and the reliance on crosslinking.[7] Crosslinking may bind other chromatin-bound proteins that transiently interact with DNA, hence limiting the amount of non-crosslinked DNA that can be recovered and assayed from the aqueous phase.[52] Thus, the overall resolution obtained from FAIRE-seq can be relatively lower than that of DNase-seq or MNase-seq[52] and with the 100,000 cell requirement,[7] the single-cell equivalents of DNase-seq[56] or MNase-seq[51] make them far more appealing alternatives.[4]
ATAC-seq
ATAC-seq is the most recently developed class of chromatin accessibility assays.[8] ATAC-seq uses a hyperactive transposase to insert transposable markers with specific adapters, capable of binding primers for sequencing, into open regions of chromatin. PCR can then be used to amplify sequences adjacent to the inserted transposons, allowing for determination of open chromatin sequences without causing a shift in chromatin structure.[8][9] ATAC-seq has been proven effective in humans, amongst other eukaryotes, including in frozen samples.[60] As with DNase-seq[56] and MNase-seq,[51] a successful single-cell version of ATAC-seq has also been developed.[61]
ATAC-seq has several advantages over MNase-seq in assessing chromatin accessibility. ATAC-seq does not rely on the variable digestion of the micrococcal nuclease, nor crosslinking or phenol-chloroform extraction.[5][9] It generally maintains chromatin structure, so results from ATAC-seq can be used to directly assess chromatin accessibility, rather than indirectly via MNase-seq. ATAC-seq can also be completed within a few hours,[9] whereas the other three techniques typically require overnight incubation periods.[5][6][7] The two major disadvantages to ATAC-seq, in comparison to MNase-seq, are the requirement for higher sequencing coverage and the prevalence of mitochondrial contamination due to non-specific insertion of DNA into both mitochondrial DNA and nuclear DNA.[8][9] Despite these minor disadvantages, use of ATAC-seq over the alternatives is becoming more prevalent.[4]
References
- ↑ 1.0 1.1 1.2 1.3 "Flexibility and constraint in the nucleosome core landscape of Caenorhabditis elegans chromatin". Genome Research 16 (12): 1505–16. December 2006. doi:10.1101/gr.5560806. PMID 17038564.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Dynamic regulation of nucleosome positioning in the human genome". Cell 132 (5): 887–98. March 2008. doi:10.1016/j.cell.2008.02.022. PMID 18329373.
- ↑ 3.0 3.1 3.2 3.3 "A non-homogeneous hidden-state model on first order differences for automatic detection of nucleosome positions". Statistical Applications in Genetics and Molecular Biology 8 (1): Article29. January 2009. doi:10.2202/1544-6115.1454. PMID 19572828.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 "Genomic methods in profiling DNA accessibility and factor localization". Chromosome Research 28 (1): 69–85. November 2019. doi:10.1007/s10577-019-09619-9. PMID 31776829.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 "Genome-wide approaches to determining nucleosome occupancy in metazoans using MNase-Seq". Chromatin Remodeling. Methods in Molecular Biology. 833. January 2012. pp. 413–9. doi:10.1007/978-1-61779-477-3_24. ISBN 978-1-61779-476-6.
- ↑ 6.0 6.1 6.2 "FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin". Genome Research 17 (6): 877–85. June 2007. doi:10.1101/gr.5533506. PMID 17179217.
- ↑ 7.0 7.1 7.2 7.3 7.4 "FAIRE (Formaldehyde-Assisted Isolation of Regulatory Elements) isolates active regulatory elements from human chromatin". Genome Research 17 (6): 877–85. June 2007. doi:10.1101/gr.5533506. PMID 17179217.
- ↑ 8.0 8.1 8.2 8.3 "Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position". Nature Methods 10 (12): 1213–8. December 2013. doi:10.1038/nmeth.2688. PMID 24097267.
- ↑ 9.0 9.1 9.2 9.3 9.4 "ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide". Current Protocols in Molecular Biology 109: 21.29.1–21.29.9. January 2015. doi:10.1002/0471142727.mb2129s109. PMID 25559105.
- ↑ "A deoxyribonuclease of Micrococcus pyogenes". Journal of the American Chemical Society 78 (18): 4642–4645. September 1956. doi:10.1021/ja01599a031.
- ↑ "Crystalline extracellular nuclease of Staphylococcus aureus". The Journal of Biological Chemistry 241 (19): 4389–90. October 1966. doi:10.1016/S0021-9258(18)99732-2. PMID 5922963.
- ↑ 12.0 12.1 "Characterization of a nuclease produced by Staphylococcus aureus". The Journal of Biological Chemistry 242 (5): 1016–20. March 1967. doi:10.1016/S0021-9258(18)96225-3. PMID 6020427.
- ↑ "Subunit structure of chromatin". Nature 251 (5472): 249–51. September 1974. doi:10.1038/251249a0. PMID 4422492. Bibcode: 1974Natur.251..249N.
- ↑ "Spheroid chromatin units (v bodies)". Science 183 (4122): 330–2. January 1974. doi:10.1126/science.183.4122.330. PMID 4128918. Bibcode: 1974Sci...183..330O.
- ↑ "Chromatin structure: a repeating unit of histones and DNA". Science 184 (4139): 868–71. May 1974. doi:10.1126/science.184.4139.868. PMID 4825889. Bibcode: 1974Sci...184..868K.
- ↑ "Structure of eukaryotic chromatin. Evaluation of periodicity using endogenous and exogenous nucleases". Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis 425 (1): 84–94. February 1976. doi:10.1016/0005-2787(76)90218-5. PMID 1247619.
- ↑ 17.0 17.1 17.2 "Periodicity and fragment size of DNA from mouse TLT hepatoma chromatin and chromatin fractions using endogenous and exogenous nucleases". Molecular and Cellular Biochemistry 19 (2): 93–112. April 1978. doi:10.1007/bf00232599. PMID 206820.
- ↑ "Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome". Cell 98 (3): 285–94. August 1999. doi:10.1016/s0092-8674(00)81958-3. PMID 10458604.
- ↑ "Removal of histone H1 exposes a fifty base pair DNA segment between nucleosomes". Biochemistry 15 (15): 3307–14. July 1976. doi:10.1021/bi00660a022. PMID 952859.
- ↑ "[Sequence analysis of oliogonucleotides by means of micrococcal nuclease]". European Journal of Biochemistry 2 (1): 102–5. July 1967. doi:10.1111/j.1432-1033.1967.tb00113.x. PMID 6079759.
- ↑ "The mitochondrial genome of wild-type yeast cells. IV. Genes and spacers". Journal of Molecular Biology 86 (4): 825–41. July 1974. doi:10.1016/0022-2836(74)90356-8. PMID 4610147.
- ↑ "Sequence analysis of the ribosome-protected bacteriophase phiX174 DNA fragment containing the gene G initiation site". Journal of Molecular Biology 92 (3): 377–93. March 1975. doi:10.1016/0022-2836(75)90287-9. PMID 1095758.
- ↑ "DNA sequence analysis. Terminal sequences of bacteriophage phi80". The Journal of Biological Chemistry 250 (12): 4607–18. June 1975. doi:10.1016/S0021-9258(19)41345-8. PMID 166999.
- ↑ "The conformation dependent hydrolysis of DNA by micrococcal nuclease". Biochimica et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis 157 (1): 114–26. March 1968. doi:10.1016/0005-2787(68)90270-0. PMID 4296058.
- ↑ "Phasing of nucleosomes in SV40 chromatin reconstituted in vitro". Journal of Biochemistry 89 (5): 1375–89. May 1981. doi:10.1093/oxfordjournals.jbchem.a133329. PMID 6168635.
- ↑ "Chromatin structure of the histone genes of D. melanogaster". Cell 23 (2): 401–9. February 1981. doi:10.1016/0092-8674(81)90135-5. PMID 6258802.
- ↑ "Detailed analysis of the nucleosomal organization of transcribed DNA in yeast chromatin". Biochemistry 20 (21): 5966–72. October 1981. doi:10.1021/bi00524a007. PMID 6272832.
- ↑ "Nucleosome phasing and micrococcal nuclease cleavage of African green monkey component alpha DNA". Proceedings of the National Academy of Sciences of the United States of America 79 (1): 118–22. January 1982. doi:10.1073/pnas.79.1.118. PMID 6275381. Bibcode: 1982PNAS...79..118M.
- ↑ "The association of human c-Ha-ras sequences with chromatin and nuclear proteins". Biochemical and Biophysical Research Communications 128 (1): 226–32. April 1985. doi:10.1016/0006-291x(85)91668-7. PMID 3885946.
- ↑ "Human IL-12(p35) gene activation involves selective remodeling of a single nucleosome within a region of the promoter containing critical Sp1-binding sites". Blood 101 (12): 4894–902. June 2003. doi:10.1182/blood-2002-09-2851. PMID 12576336.
- ↑ "Life with 6000 genes". Science 274 (5287): 546, 563–7. October 1996. doi:10.1126/science.274.5287.546. PMID 8849441. Bibcode: 1996Sci...274..546G.
- ↑ The C. Elegans Sequencing Consortium (December 1998). "Genome sequence of the nematode C. elegans: a platform for investigating biology". Science 282 (5396): 2012–8. doi:10.1126/science.282.5396.2012. PMID 9851916. Bibcode: 1998Sci...282.2012..
- ↑ "The genome sequence of Drosophila melanogaster". Science 287 (5461): 2185–95. March 2000. doi:10.1126/science.287.5461.2185. PMID 10731132. Bibcode: 2000Sci...287.2185..
- ↑ The Arabidopsis Genome Initiative (December 2000). "Analysis of the genome sequence of the flowering plant Arabidopsis thaliana". Nature 408 (6814): 796–815. doi:10.1038/35048692. PMID 11130711. Bibcode: 2000Natur.408..796T.
- ↑ "Initial sequencing and comparative analysis of the mouse genome". Nature 420 (6915): 520–62. December 2002. doi:10.1038/nature01262. PMID 12466850. Bibcode: 2002Natur.420..520W.
- ↑ International Human Genome Sequencing Consortium (October 2004). "Finishing the euchromatic sequence of the human genome". Nature 431 (7011): 931–45. doi:10.1038/nature03001. PMID 15496913. Bibcode: 2004Natur.431..931H.
- ↑ "Global nucleosome occupancy in yeast". Genome Biology 5 (9): R62. August 2004. doi:10.1186/gb-2004-5-9-r62. PMID 15345046.
- ↑ "Genome-scale identification of nucleosome positions in S. cerevisiae". Science 309 (5734): 626–630. July 2005. doi:10.1126/science.1112178. PMID 15961632. Bibcode: 2005Sci...309..626Y.
- ↑ "A high-resolution atlas of nucleosome occupancy in yeast". Nature Genetics 39 (10): 1235–44. October 2007. doi:10.1038/ng2117. PMID 17873876.
- ↑ "High-throughput mapping of the chromatin structure of human promoters". Nature Biotechnology 25 (2): 244–8. February 2007. doi:10.1038/nbt1279. PMID 17220878.
- ↑ "Independent and complementary methods for large-scale structural analysis of mammalian chromatin". Genome Research 17 (6): 928–39. June 2007. doi:10.1101/gr.5636607. PMID 17568008.
- ↑ "Genome-wide MNase hypersensitivity assay unveils distinct classes of open chromatin associated with H3K27me3 and DNA methylation in Arabidopsis thaliana". Genome Biology 21 (1): 24. February 2020. doi:10.1186/s13059-020-1927-5. PMID 32014062.
- ↑ "ATP-dependent chromatin remodeling: genetics, genomics and mechanisms". Cell Research 21 (3): 396–420. March 2011. doi:10.1038/cr.2011.32. PMID 21358755.
- ↑ "MNase titration reveals differences between nucleosome occupancy and chromatin accessibility". Nature Communications 7: 11485. May 2016. doi:10.1038/ncomms11485. PMID 27151365. Bibcode: 2016NatCo...711485M.
- ↑ 45.0 45.1 "Regulation of Nucleosome Architecture and Factor Binding Revealed by Nuclease Footprinting of the ESC Genome". Cell Reports 13 (1): 61–69. October 2015. doi:10.1016/j.celrep.2015.08.071. PMID 26411677.
- ↑ "Comparison of next-generation sequencing systems". Journal of Biomedicine & Biotechnology 2012: 251364. January 2012. doi:10.1155/2012/251364. PMID 22829749.
- ↑ "Nucleosome destabilization in the epigenetic regulation of gene expression". Nature Reviews. Genetics 9 (1): 15–26. January 2008. doi:10.1038/nrg2206. PMID 18059368.
- ↑ "Global nucleosome distribution and the regulation of transcription in yeast". Genome Biology 5 (10): 243. September 2004. doi:10.1186/gb-2004-5-10-243. PMID 15461807.
- ↑ "Fine-Resolution Mapping of TF Binding and Chromatin Interactions". Cell Reports 22 (10): 2797–2807. March 2018. doi:10.1016/j.celrep.2018.02.052. PMID 29514105.
- ↑ 50.0 50.1 50.2 50.3 "A simple method for generating high-resolution maps of genome-wide protein binding". eLife 4: e09225. June 2015. doi:10.7554/eLife.09225. PMID 26079792.
- ↑ 51.0 51.1 51.2 51.3 51.4 51.5 51.6 "Principles of nucleosome organization revealed by single-cell micrococcal nuclease sequencing". Nature 562 (7726): 281–285. October 2018. doi:10.1038/s41586-018-0567-3. PMID 30258225. Bibcode: 2018Natur.562..281L.
- ↑ 52.0 52.1 52.2 "Chromatin accessibility: a window into the genome". Epigenetics & Chromatin 7 (1): 33. November 2014. doi:10.1186/1756-8935-7-33. PMID 25473421.
- ↑ "ChIP-seq: advantages and challenges of a maturing technology". Nature Reviews. Genetics 10 (10): 669–80. October 2009. doi:10.1038/nrg2641. PMID 19736561.
- ↑ "High-resolution mapping and characterization of open chromatin across the genome". Cell 132 (2): 311–22. January 2008. doi:10.1016/j.cell.2007.12.014. PMID 18243105.
- ↑ 55.0 55.1 "Refined DNase-seq protocol and data analysis reveals intrinsic bias in transcription factor footprint identification". Nature Methods 11 (1): 73–78. January 2014. doi:10.1038/nmeth.2762. PMID 24317252.
- ↑ 56.0 56.1 56.2 "Genome-wide mapping of DNase I hypersensitive sites in rare cell populations using single-cell DNase sequencing". Nature Protocols 12 (11): 2342–2354. November 2017. doi:10.1038/nprot.2017.099. PMID 29022941.
- ↑ "The accessible chromatin landscape of the human genome". Nature 489 (7414): 75–82. September 2012. doi:10.1038/nature11232. PMID 22955617. Bibcode: 2012Natur.489...75T.
- ↑ "A map of open chromatin in human pancreatic islets". Nature Genetics 42 (3): 255–9. March 2010. doi:10.1038/ng.530. PMID 20118932.
- ↑ "Using formaldehyde-assisted isolation of regulatory elements (FAIRE) to isolate active regulatory DNA". Nature Protocols 7 (2): 256–67. January 2012. doi:10.1038/nprot.2011.444. PMID 22262007.
- ↑ "Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution". Nature Genetics 48 (10): 1193–203. October 2016. doi:10.1038/ng.3646. PMID 27526324.
- ↑ "Single-cell chromatin accessibility reveals principles of regulatory variation". Nature 523 (7561): 486–90. July 2015. doi:10.1038/nature14590. PMID 26083756. Bibcode: 2015Natur.523..486B.
 |