Biology:NBPF1
 Generic protein structure example |
Neuroblastoma breakpoint family, member 1, or NBPF1, is a protein that is encoded by the gene NBPF1 in humans. This protein is member of the neuroblastoma breakpoint family of proteins, a group of proteins that are thought to be involved in the development of the nervous system.[1]
Gene
The NBPF1 gene in humans is located on the minus strand of 1p36.3 in humans and is 51179 base pairs long including exons and introns. It is located between the protein coding genes NECAP2 and CROCC. NBPF1 is one of the 26 known members of the Neuroblastoma Breakpoint Family genes and pseudogenes. The NBPF2 pseudogene and NBPF3 gene are the most similar genes located close to NBPF1 and they reside on the chromosomal location 1p36.12. Most members of the NBPF gene family are located on chromosomal location 1q21.1-1q23.3 in humans, and these genes are more similar to each other in sequence than they are to NBPF1.[2]
Transcript
The transcript for NBPF1 in humans is a 6183 base pair mRNA that is made of 28 exons. There are more than 14 alternative splicing forms of NBPF1 predicted, but only seven of the splice forms have been observed. Out of all of the possible transcripts, only two are known to code proteins. One of these transcripts is 1139 amino acids long with 23 coding exons, while and the other is 1095 amino acids long and 23 coding exons. The noncoding transcripts are processed, but their function is unknown.[1][3][4]
Protein
NBPF1 is a 1214 amino acid long protein in humans that weighs 139 kD and has an isoelectric point of around 5. A feature about this protein's composition is that it is much richer than most other proteins in both glutamine and glutamic acid residues. Additionally, it contains amino acid repeats that are present in humans, other primates, and even armadillos.[5] Another feature is that the NBPF1 protein contains residues that are predicted to have post-translational modifications, including glycation, N-linked glycosylation, O-GlcNAc attachment, O-linked glycosylation, Phosphorylation, and Sumoylation.[6] The two most important domain types in the NBPF1 protein are the coiled coil domains and DUF1220 domains. NBPF1 contains three coiled coil domains and nine DUF1220 domains in humans. The coiled coil domains are 60-100 amino acids long, while the DUF1220 domains are approximately 65 base pairs long with high sequence similarity.[7]
Interactions
Human NBPF1 has been shown to interact with Ubiquitin C via both protein complex immunoprecipitation[8] and affinity chromatography.[9] Two-hybrid screening assays have shown that NBPF1 interacts with the coiled coil domains of CBY1, a repressor for the Beta-catenin, a protein that is involved in Wnt signaling for cell proliferation.[10] Additionally, two hybrid screen have shown that NBPF1 interacted with bacterial proteins, such as an Oxidoreductase iron/ascorbate family protein from the bacterium Francisella tularensis and an uncharacterized protein from the bacterium Bacillus anthracis.[11]
Functional and clinical significance
Although the function of the NBPF1 protein in unknown, its physical and chemical properties can give insight about its function. Like other NBPF proteins, the NBPF1 protein product contains a repeated domain called DUF1220, a domain of unknown function that is thought to be related to human brain complexity.[12] First, NBPF1 is predicted to be a nuclear protein, as it contains positively charged nuclear localization signals. These nuclear localization signals in NBPF1 and a conserved DNA binding domain similar to the transcription factor STAT3/dna complex or STAT1/dna complex suggests that it could act as a transcription factor[13]
Second, genes like NBPF1 with DUF1220 genes are expressed during human neurogenesis. The number of DUF1220 domains present in the human genome correlates with both brain size and the amount of neurons present in the brain.[14] Additionally, higher copy numbers of the DUF1220 domain are associated with increasing Autism severity, which often results from an excess of neurons that do not under synaptic pruning.[15]
The NBPF1 protein is also found to be disrupted by a chromosomal translocation between chromosomes 1 and 17 with in some cases of human neuroblastoma.[16] Additional studies show that NBPF1 is possibly a tumor suppressor gene because adding it to cell cultures lowers the incidence of foci formation. Additionally, NBPF1 contains three coiled coil structures are commonly involved in oligomerization with other proteins.[17] Based on the interactions listed above, NBPF1 is shown to interact with the coiled coil domain of CBY1, which represses Wnt signaling.[16] Aberrant Wnt signaling in the brain is a common cause of tumor growth and drug resistance in neuroblastomas,[18] further suggesting that NBPF1 could act as a tumor suppressor gene in the brain if it directly affects this pathway.
NBPF1 seems to be involved in proliferation during human neurogenesis, and growth suppression during adulthood. Showing that the DUF1220 and coiled coil domains may be important during different life stages. The DUF1220 domains are important in neurogenesis, while the coiled coil domains involved with the binding of cell proliferation inhibitors. Although there are no known differences between Autism and neuroblastoma rates are known, a study has shown the existence of comorbid microcephaly and neuroblastoma conditions, although more research is needed to show this.[19] Based on these predictions, the lack of NBPF1 during could prevent fetal neurogenesis and postnatal tumor suppression in the brain, although this connection is not well understood.
Expression
NBPF1 is ubiquitously expressed in all tissues in humans, but shows the highest levels of expression in the bone marrow, skeletal muscle, brain, and spinal cord. It is expressed at slightly lower levels in other tissues such as the pancreas, kidney, and lung.[20] In the brain, NBPF1 expression is the highest in the frontal, temporal, and parietal lobes, and it lowest in the ventricles and cerebellum.[21] Based on protein composition, NBPF1 in humans and its orthologs in related species is most likely to be localized in the nucleus.[22]
Predicted Localization of NBPF1 and its Orthologs[22]
| Species | %Nuclear | %Vacuolar | %Cytoplasmic | %Cytoskeletal |
|---|---|---|---|---|
| Human | 87.0 | 4.3 | 4.3 | 4.3 |
| Macaque | 65.2 | 4.3 | 17.4 | 4.3 |
| Baboon | 82.6 | 0.0 | 13.0 | 0.0 |
| Cow | 65.2 | 0.0 | 17.4 | 0.0 |
| Armadillo | 65.2 | 0.0 | 13.0 | 0.0 |
Expression studies have shown changes in NBPF1 under different experimental conditions in vitro. First, the depletion of nervous system transcription factor SOX11 causes a slight increase in NBPF1 expression.[23] Additionally, the inactivation of Far upstream element-binding protein 1 causes a decrease in NBPF1, while the inactivation of Far Upstream Binding Elements 2 and 3 causes an increase in NBPF1 expression.[24] Far upstream binding elements are involved in transcriptional regulation using gene enhancers, each having different binding sites.[25] The overexpression of CLDN1, a protein that forms tight junctions such as those of the blood–brain barrier, causes a sharp decline in NBPF1 expression[26]
Homology and evolution
Orthologs
Although NBPF1 itself only exists in primates, a wide variety of NBPF like protein orthologs exist in other mammals such as cattle, felines, and cetaceans. In non-primate mammals, the gene sequences of NBPF-like genes have little similarity to the primate NBPF genes. These genes appear to be entirely absent in model mammals such as mice and rats. The large amount of NBPF genes in the human genome is most likely due to recent duplications because all of the NBPF genes are so similar and repetitive that they easily recombine with each other, causing duplications. Variation in the number of repetitive sequences in the NBPF genes also varies even within humans.[27] DUF1220 domains also vary greatly from humans in other species in their NBPF proteins. The further away a species is from humans, the fewer DUF1220 domains the species has. Humans have on average 272 DUF1220 domains in their NBPF genes, while chimpanzees have 125, macaques have 35, and dolphins only have 4.[28]
Selected Orthologs of NBPF1[29][30]
| Species | Common name | Time of divergence from humans (mya) | NCBI accession number | Sequence length (amino acids) | Protein similarity to human NBPF1 | mRNA similarity to human NBPF1 |
|---|---|---|---|---|---|---|
| Homo sapiens | Human | 0 | AAX85114.1 | 1214 | 100% | 100% |
| Pan troglodytes | Chimpanzee | 6.3 | XP_009439437.1 | 656 | 93% | 98% |
| Macaca fascicularis | Crab-eating macaque | 29 | XP_005544713.1 | 1173 | 77% | 87% |
| Tursiops truncates | Bottle-nosed dolphin | 94.2 | XP_004329243.1 | 888 | 40% | 37% |
| Bos taurus | Cattle | 94.2 | XP_005197798.1 | 633 | 39% | 36% |
| Felis catus | Domestic cat | 94.2 | XP_011283477.1 | 813 | 38% | 35% |
| Sus scrofa | Pig | 94.2 | XP_005653139.1 | 567 | 36% | 40% |
| Dasypus novemcinctus | Nine-banded armadillo | 104.2 | XP_004469026 | 934 | 30% | 34% |
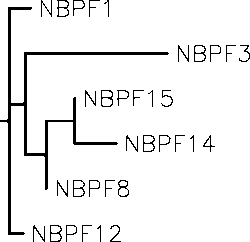
Paralogs
The paralogs for NBPF1 are other members of the NBPF protein family. The highest similarity between these paralogs further shows evidence of gene duplication during human evolution.
Selected Paralogs of NBPF1
| Gene name | NCBI accession number | Sequence length (amino acids) | Protein sequence identity | Protein sequence similarity |
|---|---|---|---|---|
| NBPF1 | NP_060410.2 | 1214 | 100% | 100% |
| NBPF12 | NP_001265070.1 | 1457 | 100% | 93% |
| NBPF8 | NP_001032590.2 | 942 | 100% | 93% |
| NBPF14 | NP_056198.2 | 2819 | 100% | 92% |
| NBPF15 | NP_775909.2 | 670 | 98% | 93% |
| NBPF3 | NP_115640.1 | 633 | 100% | 75% |
References
- ↑ 1.0 1.1 "NBPF1 neuroblastoma breakpoint family, member 1 [Homo sapiens (human)] - Gene - NCBI". ncbi.nlm.nih.gov. https://www.ncbi.nlm.nih.gov/gene/55672.
- ↑ "Neuroblastoma breakpoint family | HUGO Gene Nomenclature Committee". https://www.genenames.org/genefamilies/NBPF.
- ↑ "Gene: NBPF1 (ENSG00000219481) - Summary - Homo_sapiens - Ensembl genome browser 106". http://useast.ensembl.org/Homo_sapiens/Gene/Summary?db=core;g=ENSG00000219481;r=1:16562319-16613562;t=ENST00000430580;redirect=no.
- ↑ "NBPF1 - Neuroblastoma breakpoint family member 1 - Homo sapiens (Human)". uniprot.org. https://www.uniprot.org/uniprot/Q3BBV0.
- ↑ Brendel, V; Bucher, P; Nourbakhsh, I.R.; Blaisdell, B.E.; Karlin, S (1992). "Methods and algorithms for statistical analysis of protein sequences". Proceedings of the National Academy of Sciences of the United States of America (National Academy of Sciences) 89 (6): 2002–2006. doi:10.1073/pnas.89.6.2002. PMID 1549558. Bibcode: 1992PNAS...89.2002B.
- ↑ "ExPASy Proteomics: Post-Translational Modification Tools". http://www.expasy.org/proteomics/post-translational_modification.
- ↑ "Q3BBV0 - NBPF1_HUMAN". https://www.uniprot.org/uniprot/Q3BBV0.
- ↑ "Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes?". The EMBO Journal 32 (4): 552–65. February 2013. doi:10.1038/emboj.2012.354. PMID 23314748.
- ↑ "Systematic and quantitative assessment of the ubiquitin-modified proteome". Molecular Cell 44 (2): 325–40. October 2011. doi:10.1016/j.molcel.2011.08.025. PMID 21906983.
- ↑ "Chibby interacts with NBPF1 and clusterin, two candidate tumor suppressors linked to neuroblastoma". Experimental Cell Research 316 (7): 1225–33. April 2010. doi:10.1016/j.yexcr.2010.01.019. PMID 20096688.
- ↑ "The human-bacterial pathogen protein interaction networks of Bacillus anthracis, Francisella tularensis, and Yersinia pestis". PLOS ONE 5 (8): e12089. August 2010. doi:10.1371/journal.pone.0012089. PMID 20711500. Bibcode: 2010PLoSO...512089D.
- ↑ "DUF1220 domains, cognitive disease, and human brain evolution". Cold Spring Harbor Symposia on Quantitative Biology 74: 375–82. 2009. doi:10.1101/sqb.2009.74.025. PMID 19850849.
- ↑ "NBPF is a potential DNA-binding transcription factor that is directly regulated by NF-κB". The International Journal of Biochemistry & Cell Biology 45 (11): 2479–90. November 2013. doi:10.1016/j.biocel.2013.07.022. PMID 23939288.
- ↑ "DUF1220 protein domains drive proliferation in human neural stem cells and are associated with increased cortical volume in anthropoid primates". Brain Structure & Function 220 (5): 3053–60. September 2015. doi:10.1007/s00429-014-0814-9. PMID 24957859.
- ↑ "DUF1220 dosage is linearly associated with increasing severity of the three primary symptoms of autism". PLOS Genetics 10 (3): e1004241. March 2014. doi:10.1371/journal.pgen.1004241. PMID 24651471.
- ↑ 16.0 16.1 "A constitutional translocation t(1;17)(p36.2;q11.2) in a neuroblastoma patient disrupts the human NBPF1 and ACCN1 genes". PLOS ONE 3 (5): e2207. May 2008. doi:10.1371/journal.pone.0002207. PMID 18493581. Bibcode: 2008PLoSO...3.2207V.
- ↑ "Coiled coils: a highly versatile protein folding motif". Trends in Cell Biology 11 (2): 82–8. February 2001. doi:10.1016/s0962-8924(00)01898-5. PMID 11166216.
- ↑ "Wnt signaling: multiple functions in neural development". Cellular and Molecular Life Sciences 62 (10): 1100–8. May 2005. doi:10.1007/s00018-005-4552-2. PMID 15928805. http://doc.rero.ch/record/318183/files/18_2005_Article_4552.pdf.
- ↑ "Bilateral neuroblastoma in situ associated with microcephaly". Journal of Korean Medical Science 8 (2): 99–103. April 1993. doi:10.3346/jkms.1993.8.2.99. PMID 8397936.
- ↑ "GDS424 / 59465_r_at / NBPF1, Normal human tissue expression profiling". https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS424:59465_r_at.
- ↑ "Allen Brain Atlas: Human Brain Microarray". http://human.brain-map.org/microarray/search/show?exact_match=false&search_term=%22NBPF1%22&search_type=gene.
- ↑ 22.0 22.1 Nakai, Kenta. "PSORT II". http://www.genscript.com/psort/psort2.html.
- ↑ "Neural transcription factor SOX11 depletion effect on mantle cell lymphoma cell line". https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS4801:236273_at.
- ↑ "Far-upstream element binding protein inactivation". https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS2573:1755150_1.
- ↑ "The far upstream element-binding proteins comprise an ancient family of single-strand DNA-binding transactivators". The Journal of Biological Chemistry 271 (49): 31679–87. December 1996. doi:10.1074/jbc.271.49.31679. PMID 8940189.
- ↑ "Claudin-1 overexpression effect on lung adenocarcinoma cell line". https://www.ncbi.nlm.nih.gov/geo/tools/profileGraph.cgi?ID=GDS3510:236273_at.
- ↑ "A novel gene family NBPF: intricate structure generated by gene duplications during primate evolution". Molecular Biology and Evolution 22 (11): 2265–74. November 2005. doi:10.1093/molbev/msi222. PMID 16079250.
- ↑ "DUF1220-domain copy number implicated in human brain-size pathology and evolution". American Journal of Human Genetics 91 (3): 444–54. September 2012. doi:10.1016/j.ajhg.2012.07.016. PMID 22901949.
- ↑ "NCBI BLAST". http://blast.ncbi.nlm.nih.gov/Blast.cgi.
- ↑ "TimeTree: a public knowledge-base of divergence times among organisms". Bioinformatics 22 (23): 2971–2. December 2006. doi:10.1093/bioinformatics/btl505. PMID 17021158.
 |