Biology:YadA bacterial adhesin protein domain
| YadA bacterial adhesin protein domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
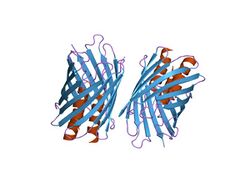 The beta barrel structure found in the C-terminus of the bacterial adhesin protein domain, YadA [1] | |||||||||
| Identifiers | |||||||||
| Symbol | YadA | ||||||||
| Pfam | PF03895 | ||||||||
| Pfam clan | CL0327 | ||||||||
| InterPro | IPR005594 | ||||||||
| |||||||||
In molecular biology, YadA is a protein domain which is short for Yersinia adhesin A. These proteins have strong sequence and structural homology, particularly at their C-terminal end. The function is to promote their pathogenicity and virulence in host cells, though cell adhesion. YadA is found in three pathogenic species of Yersinia, Y. pestis, Y. pseudotuberculosis, and Y. enterocolitica. The YadA domain is encoded for by a virulence plasmid in Yersinia, which encodes a type-III secretion (T3S) system consisting of the Ysc injectisome and the Yop effectors.[1]
Function
Essentially, the main function of the YadA domain is to help cell adhesion and to increase virulence. YadA is a collagen-binding outer membrane protein. It forms the fibrillar matrix on the bacterial cell surface. This aids cell attachment and helps the bacteria invade eukaryotic cells. Additionally, by forming the fibrillar matrix, the YadA domain protects the bacteria by facilitating agglutination resistance, serum resistance, complement inactivation and phagocytosis resistance.
The importance of adhesins to YadA function and Yersinia survival is huge. Attachment further allows more interactions and increase of biofilm formation to aid bacterial colonization. In Yersinia, it helps initiate the infectious process in host cells and are critical virulence factors. Additionally, bacteria have the ability to regulate adhesion expression, meaning that when Yersinia no longer requires YadA, it can be turned off.[2] Furthermore, YadA expression is mainly temperature regulated, at 37 degrees Celsius. It also has two molecular regulators: an activator, VirF and a repressor, YmoA.[3]
Substrate adhesion
The YadA protein domain adheres to the following substrates:[1]
- epithelial cells
- extracellular matrix
- collagen
- cellular fibronectin
- laminin
Protein domains in YadA
C terminal domain
The C-terminal domain consists of 120 amino acids which belong to a family of surface-exposed bacterial proteins. The YadA C-terminal domain has a particular function in translocating the trimeric N-terminal passenger domain to the exterior of the membrane and is also responsible for trimerisation.[4]
Structure
C-terminal domain structure
The C-terminal region is a transmembrane region which consists of 4 beta strands which form trimers in the outer membrane.[5] The C-terminal contains 9 amino acids which alternate hydrophobic amino acids ending in F (Phenylalanine) or W (Tryptophan), this composes a targeting motif for the outer membrane of the Gram-negative cell membrane. This region is important for oligomerisation.[6] The C-terminal domain helps to build the beta barrel pore in the outer membrane.
YadA protein structure
YadA is a homotrimeric outer membrane protein which forms part of the fibrillar matrix. Simplistically, this means the protein is made of three of the same subunits, on the outer surface of the membrane. The surface is entirely covered in the YadA lollipop structures. made of a short C-terminal membrane anchor, an 18 nm long coiled-coil stem and a 5 nm long N-terminal globular head structure consisting of a left-handed parallel beta roll. YadA is an example of an oligomeric coiled-coil adhesion (Oca). The Oca protein families are a subset of autotransporters, also known as the type Vc or trimeric autotransporters.[1]
Trimerization is thought to involve the coiled-coil stem and the C-terminal membrane anchor, which forms a 12-strand beta-barrelfrom the four transmembrane beta-strands of the three monomers. This beta-barrel would form a pore-like structure through which the N-terminal head and coiled helical domains of the three monomer chains exit to the cell surface. The YadA protein domain, is a form of trimeric autotransporter adhesins (TAAs). Each TAA must consist of a head, stalk and a beta-barrel membrane anchor.[7]
History
YadA, an adhesin from Yersinia, was the first member of this family to be characterised. UspA2 from Moraxella was second. The Eib immunoglobulin-binding proteins from Escherichia coli were third, followed by the DsrA proteins of Haemophilus ducreyi, amongst others.
See also
Trimeric Autotransporter Adhesins (TAA)
References
- ↑ 1.0 1.1 1.2 "Oligomeric coiled-coil adhesin YadA is a double-edged sword.". PLOS ONE 5 (12): e15159. 2010. doi:10.1371/journal.pone.0015159. PMID 21170337. Bibcode: 2010PLoSO...515159C.
- ↑ Thanassi DG (2011). "The long and the short of bacterial adhesion regulation.". J Bacteriol 193 (2): 327–8. doi:10.1128/JB.01345-10. PMID 21097622.
- ↑ "Bacterial cell surface structures in Yersinia enterocolitica.". Arch Immunol Ther Exp (Warsz) 60 (3): 199–209. 2012. doi:10.1007/s00005-012-0168-z. PMID 22484801.
- ↑ "Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica.". J Bacteriol 190 (14): 5031–43. 2008. doi:10.1128/JB.00161-08. PMID 18487327.
- ↑ "Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA.". J Bacteriol 185 (13): 3735–44. 2003. doi:10.1128/jb.185.13.3735-3744.2003. PMID 12813066.
- ↑ "Nonimmune binding of human immunoglobulin A (IgA) and IgG Fc by distinct sequence segments of the EibF cell surface protein of Escherichia coli". Infect. Immun. 69 (12): 7293–303. December 2001. doi:10.1128/IAI.69.12.7293-7203.2001. PMID 11705900.
- ↑ "C-terminal amino acid residues of the trimeric autotransporter adhesin YadA of Yersinia enterocolitica are decisive for its recognition and assembly by BamA.". Mol Microbiol 78 (4): 932–46. 2010. doi:10.1111/j.1365-2958.2010.07377.x. PMID 20815824.
 |

