Chemistry:Ethyl protocatechuate
From HandWiki
Revision as of 10:42, 26 July 2021 by imported>AstroAI (change)
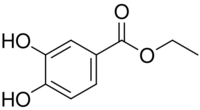
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl 3,4-dihydroxybenzoate | |
| Other names
Ethyl ester of 3,4-dihydroxybenzoic acid
3,4-Dihydroxybenzoic acid ethyl ester EDHB Ethyl-3,4-dihydroxybenzoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10O4 | |
| Molar mass | 182.175 g·mol−1 |
| Appearance | White or pale brownish yellow, crystalline powder; odorless or has a faint
phenol-like odour |
| Melting point | 132 to 135 °C (270 to 275 °F; 405 to 408 K) |
| Boiling point | 357 to 358 °C (675 to 676 °F; 630 to 631 K)[2] |
| Insoluble in water; soluble in ethanol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Ethyl protocatechuate is a phenolic compound. It can be found in the peanut seed testa.[3][4] It is also present in wine.[5] It is the ethylic ester of protocatechuic acid.
The compound is a prolyl 4-hydroxylase inhibitor[6] and can be used to protect the myocardium.[7]
See also
References
- ↑ Ethyl protocatechuate on FAO website
- ↑ Ethyl protocatechuate on www.thegoodscentscompany.com
- ↑ Huang, S. C.; Yen, G. C.; Chang, L. W.; Yen, W. J.; Duh, P. D. (2003). "Identification of an Antioxidant, Ethyl Protocatechuate, in Peanut Seed Testa". Journal of Agricultural and Food Chemistry 51 (8): 2380–2383. doi:10.1021/jf0210019. PMID 12670184.
- ↑ Yen, W. J.; Chang, L. W.; Duh, P. D. (2005). "Antioxidant activity of peanut seed testa and its antioxidative component, ethyl protocatechuate". LWT - Food Science and Technology 38 (3): 193. doi:10.1016/j.lwt.2004.06.004.
- ↑ Baderschneider, B.; Winterhalter, P. (2001). "Isolation and Characterization of Novel Benzoates, Cinnamates, Flavonoids, and Lignans from Riesling Wine and Screening for Antioxidant Activity". Journal of Agricultural and Food Chemistry 49 (6): 2788–2798. doi:10.1021/jf010396d. PMID 11409967.
- ↑ Wang, J.; Buss, J. L.; Chen, G.; Ponka, P.; Pantopoulos, K. (2002). "The prolyl 4-hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate generates effective iron deficiency in cultured cells". FEBS Letters 529 (2–3): 309–312. doi:10.1016/S0014-5793(02)03389-6. PMID 12372619.
- ↑ Philipp, S.; Cui, L.; Ludolph, B.; Kelm, M.; Schulz, R.; Cohen, M. V.; Downey, J. M. (2005). "Desferoxamine and ethyl-3,4-dihydroxybenzoate protect myocardium by activating NOS and generating mitochondrial ROS". AJP: Heart and Circulatory Physiology 290 (1): H450–H457. doi:10.1152/ajpheart.00472.2005. PMID 16155105.
 |

