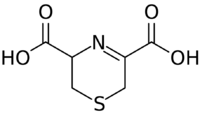
Chemistry:Lanthionine ketimine
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,6-Dihydro-2H-1,4-thiazine-3,5-dicarboxylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C6H7NO4S | |
| Molar mass | 189.19 g·mol−1 |
| Appearance | White powder |
| Melting point | 160 °C (320 °F; 433 K) (decomposes) |
| 30 g/L | |
| Hazards | |
| Main hazards | irritation |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lanthionine ketimine (3,4-dihydro-2H-1,3-thiazine-3,5-dicarboxylic acid) is a naturally occurring sulfur amino acid metabolite found in the mammalian brain and central nervous system (CNS).[1][2]
Background
Lanthionine ketimine was recognized as a natural metabolite as early as 1983 by Dorianno Cavallini, who published regarding its synthesis and chemical properties.[1][2] Cavallini and others showed that lanthionine ketimine forms from alternative reactions of the transsulfuration pathway enzyme cystathionine-β-synthase, which normally condenses the amino acids homocysteine and serine to form cystathionine. In an alternate pathway, cysteine and serine (or two equivalents of cysteine) condense to form lanthionine.[2][3][4][5] The product of these transformations is lanthionine or cystathionine ketimine,[2] respectively.
Additional sources of lanthionine ketimine have been proposed. Lanthionine ketimine also binds the brain protein lanthionine synthase-like protein-1 (LANCL1), a glutathione-binding protein of uncertain function.[5][6] It has been hypothesized, but not proved, that LANCL1 might catalyze formation of glutathione-lanthionine conjugates in a pathway leading to lanthionine ketimine.[5]
Lanthionine ketimine and a synthetic, cell-penetrating ester derivative called lanthionine ketimine-5-ethyl ester (LKE) potentiate growth factor-dependent extension of neuron processes (neurites) in cell culture.[6][7] This neurotrophic activity may occur through interaction of lanthionine ketimine with a protein called collapsin response protein-2 (CRMP2, also known as dihydropyrimidinase-like protein-2 or DPYSL2). Normally CRMP2 functions to promote or inhibit neurite growth. Lanthionine ketimine interacts with CRMP2 in affinity proteomics experiments and alters CRMP2 binding to other proteins in brain lysate preparations.[6]
Beside its neurotrophic effects, lanthionine ketimine and its ester LKE protect neurons against oxidative stress[6] and inhibit the activation of microglia (brain macrophages) triggered by exposure to inflammatory cytokines. Administration of LKE to the SOD1G93A mouse model of the motor neuron disease amyotrophic lateral sclerosis (ALS), slows progression of paralytic disease in this mouse.[5]
Preparation
Lanthionine ketimine or its ethyl esters can be synthesized by condensation of cysteine derivatives (e.g. L-cysteine-ethyl ester hydrochloride) with 3-bromopyruvic acid or derivatives in water, followed by filtration and thorough aqueous washing of the precipitate. When dried, the precipitate can be resolubilized in aqueous medium by slow titration with sodium hydroxide (NaOH) or other base.[6][8]
References
- ↑ Cavallini, D.; Ricci, G.; Federri, G. (1983) The ketimine derivatives of thialysine, lanthionine, cystathionine, cysteine: Preparation and properties. In Sulfur Amino Acids: Biochemical and Clinical Aspects, Alan R. Liss Inc., pp. 355–364
- ↑ 2.0 2.1 2.2 Cavallini, Doriano; Ricci, Giorgio; Dupre, Silvestro; Pecci, Laura; Costa, Mara; Matarese, Rosa M.; Pensa, Bernardo; Antonucci, Antonio et al. (1991). "Sulfur-containing cyclic ketimines and imino acids. A novel family of endogenous products in the search for a role". European Journal of Biochemistry 202 (2): 217–223. doi:10.1111/j.1432-1033.1991.tb16365.x. PMID 1761027.
- ↑ Hensley, Kenneth; Gabbita, S. Prasad; Venkova, Kalina; Hristov, Alexandar; Johnson, Ming F.; Eslami, Pirooz; Harris-White, Marni E. (2013). "A Derivative of the Brain Metabolite Lanthionine Ketimine Improves Cognition and Diminishes Pathology in the 3×Tg-AD Mouse Model of Alzheimer Disease". Journal of Neuropathology & Experimental Neurology 72 (10): 955–969. doi:10.1097/NEN.0b013e3182a74372. PMID 24042198.
- ↑ Cooper, Arthur J.L. (2004). "The role of glutamine transaminase K (GTK) in sulfur and α-keto acid metabolism in the brain, and in the possible bioactivation of neurotoxicants". Neurochemistry International 44 (8): 557–577. doi:10.1016/j.neuint.2003.12.002. PMID 15016471.
- ↑ 5.0 5.1 5.2 5.3 Singh, Sangita; Padovani, Dominique; Leslie, Rachel A.; Chiku, Taurai; Banerjee, Ruma (2009). "Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-sulfuration Reactions". Journal of Biological Chemistry 284 (33): 22457–22466. doi:10.1074/jbc.M109.010868. PMID 19531479.
- ↑ 6.0 6.1 6.2 6.3 6.4 Hensley, Kenneth; Venkova, Kalina; Christov, Alexandar (2010). "Emerging Biological Importance of Central Nervous System Lanthionines". Molecules 15 (8): 5581–5594. doi:10.3390/molecules15085581. PMID 20714314.
- ↑ Hensley, K.; Christov, A.; Kamat, S.; Zhang, X. C.; Jackson, K. W.; Snow, S.; Post, J. (2010). "Proteomic Identification of Binding Partners for the Brain Metabolite Lanthionine Ketimine (LK) and Documentation of LK Effects on Microglia and Motoneuron Cell Cultures". Journal of Neuroscience 30 (8): 2979–2988. doi:10.1523/JNEUROSCI.5247-09.2010. PMID 20181595.
- ↑ Koch, Janc; Zhang, Jian-Nan (2017). "Collapsin response mediator protein-2 plays a major protective role in acute axonal degeneration". Neural Regeneration Research 12 (5): 692–695. doi:10.4103/1673-5374.206631. PMID 28616018.
 |

